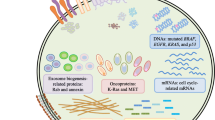
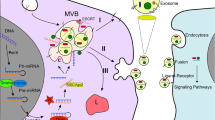
Abstract
Tumor cell-derived vesicles are released by tumor cells, have a phospholipid bilayer, and are widely distributed in various biological fluids. In recent years, it has been found that tumor cell-derived vesicles contain proteins, metabolites and nucleic acids and can be delivered to recipient cells to perform their physiological functions, such as mediating specific intercellular communication, activating or inhibiting signaling pathways, participating in regulating the modulation of tumor microenvironment and influencing tumor development, which can be used for early detection and diagnosis of cancer. In addition, tumor cell-derived vesicles exhibit multiple properties in tumor therapeutic applications and may serve as a new class of delivery systems. In this review, we elaborate on the application of tumor cell-derived vesicles in oncology therapy.



Similar content being viewed by others
References
Lv TR, Hu HJ, Regmi P, Liu F, Li FY. Sarcomatoid hepatocellular carcinoma versus conventional hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-03949-8.
Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis(2006) 27(2):262–8. https://doi.org/10.1093/carcin/bgi147.
Rossi GR, Trindade ES, Souza-Fonseca-Guimaraes F. Tumor microenvironment-associated extracellular matrix components regulate Nk cell function. Front Immunol. 2020;11:73. https://doi.org/10.3389/fimmu.2020.00073.
Fang F, Zhang T, Li Q, Chen X, Jiang F, Shen X. The tumor immune-microenvironment in gastric cancer. Tumori. 2022. https://doi.org/10.1177/03008916211070051.
Giordano C, La Camera G, Gelsomino L, Barone I, Bonofiglio D, Ando S, et al. The biology of exosomes in breast cancer progression: dissemination, immune evasion and metastatic colonization. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12082179.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (Misev2018): a position statement of the international society for extracellular vesicles and update of the Misev 2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750.
Ma J, Zhang H, Tang K, Huang B. Tumor-derived microparticles in tumor immunology and immunotherapy. Eur J Immunol. 2020;50(11):1653–62. https://doi.org/10.1002/eji.202048548.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. https://doi.org/10.1038/nri855.
Anand S, Samuel M, Mathivanan S. Exomeres: a new member of extracellular vesicles family. Subcell Biochem. 2021;97:89–97. https://doi.org/10.1007/978-3-030-67171-6_5.
Lu L, Huang J, Mo J, Da X, Li Q, Fan M, et al. Exosomal lncrna Tug1 from cancer-associated fibroblasts promotes liver cancer cell migration, invasion, and glycolysis by regulating the Mir-524–5p/Six1 Axis. Cell Mol Biol Lett. 2022;27(1):17. https://doi.org/10.1186/s11658-022-00309-9.
Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl). 2013;91(4):431–7. https://doi.org/10.1007/s00109-013-1020-6.
Scavo MP, Depalo N, Tutino V, De Nunzio V, Ingrosso C, Rizzi F, et al. Exosomes for diagnosis and therapy in gastrointestinal cancers. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21010367.
Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X. Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer. 2019;18(1):41. https://doi.org/10.1186/s12943-019-1001-7.
Wang S, Wang J, Wei W, Ma G. Exosomes: the indispensable messenger in tumor pathogenesis and the rising star in antitumor applications. Adv Biosyst. 2019;3(5):e1900008. https://doi.org/10.1002/adbi.201900008.
Kim YS, Ahn JS, Kim S, Kim HJ, Kim SH, Kang JS. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diverse biomedical fields. Korean J Physiol Pharmacol. 2018;22(2):113–25. https://doi.org/10.4196/kjpp.2018.22.2.113.
Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17(1):146. https://doi.org/10.1186/s12943-018-0898-6.
Wang S, Xu M, Li X, Su X, Xiao X, Keating A, et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 2018;11(1):82. https://doi.org/10.1186/s13045-018-0625-1.
Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–68. https://doi.org/10.1016/j.bbcan.2019.04.004.
Tian F, Zhang S, Liu C, Han Z, Liu Y, Deng J, et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun. 2021;12(1):2536. https://doi.org/10.1038/s41467-021-22913-7.
Srivastava A, Amreddy N, Razaq M, Towner R, Zhao YD, Ahmed RA, et al. Exosomes as theranostics for lung cancer. Adv Cancer Res. 2018;139:1–33. https://doi.org/10.1016/bs.acr.2018.04.001.
Mannavola F, D’Oronzo S, Cives M, Stucci LS, Ranieri G, Silvestris F, et al. Extracellular vesicles and epigenetic modifications are hallmarks of melanoma progression. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms21010052.
Kugeratski FG, Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. Febs J. 2021;288(1):10–35. https://doi.org/10.1111/febs.15558.
Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4(6):910–21. https://doi.org/10.1039/c5bm00583c.
Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. https://doi.org/10.1016/j.ymeth.2015.02.019.
Wang JM, Li YJ, Wu JY, Cai JX, Wen J, Xiang DX, et al. Comparative evaluation of methods for isolating small extracellular vesicles derived from pancreatic cancer cells. Cell Biosci. 2021;11(1):37. https://doi.org/10.1186/s13578-021-00550-3.
Lane RE, Korbie D, Anderson W, Vaidyanathan R, Trau M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep. 2015;5:7639. https://doi.org/10.1038/srep07639.
Dai Y, Gao X. Inhibition of cancer cell-derived exosomal Microrna-183 suppresses cell growth and metastasis in prostate cancer by upregulating Tpm1. Cancer Cell Int. 2021;21(1):145. https://doi.org/10.1186/s12935-020-01686-x.
Yang L, Wu XH, Wang D, Luo CL, Chen LX. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8(4):1272–8. https://doi.org/10.3892/mmr.2013.1634.
Deng M, Yuan H, Liu S, Hu Z, Xiao H. Exosome-transmitted Linc00461 promotes multiple myeloma cell proliferation and suppresses apoptosis by modulating microrna/Bcl-2 expression. Cytotherapy. 2019;21(1):96–106. https://doi.org/10.1016/j.jcyt.2018.10.006.
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. https://doi.org/10.1016/j.canlet.2011.10.002.
Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microrna-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235(5):4734–45. https://doi.org/10.1002/jcp.29351.
Xu H, Zhao G, Zhang Y, Jiang H, Wang W, Zhao D, et al. Mesenchymal stem cell-derived exosomal microrna-133b suppresses glioma progression via wnt/beta-catenin signaling pathway by targeting Ezh2. Stem Cell Res Ther. 2019;10(1):381. https://doi.org/10.1186/s13287-019-1446-z.
Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from Mir-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871. https://doi.org/10.1016/j.lfs.2020.118871.
Xu Y, Liu N, Wei Y, Zhou D, Lin R, Wang X, et al. Anticancer effects of Mir-124 delivered by Bm-Msc derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging (Albany NY). 2020;12(19):19660–76. https://doi.org/10.18632/aging.103997.
de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. https://doi.org/10.1038/nrc1782.
Jing L, Hua X, Yuanna D, Rukun Z, Junjun M. Exosomal Mir-499a-5p inhibits endometrial cancer growth and metastasis via targeting Vav3. Cancer Manag Res. 2020;12:13541–52. https://doi.org/10.2147/CMAR.S283747.
Rashed MH, Bayraktar E, Helal GK, Abd-Ellah MF, Amero P, Chavez-Reyes A, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18030538.
Yang EL, Wang X, Gong ZY, Yu M, Wu HW, Zhang DS. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Tar. 2020. https://doi.org/10.1038/s41392-020-00359-5.
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. https://doi.org/10.1038/s41392-020-00261-0.
Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22(4):342–9. https://doi.org/10.1016/j.semcancer.2012.02.005.
Zhao XY, Wu DL, Ma XD, Wang JL, Hou WJ, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020. https://doi.org/10.1016/j.biopha.2020.110237.
Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. https://doi.org/10.1186/s13045-019-0760-3.
Baig MS, Roy A, Rajpoot S, Liu D, Savai R, Banerjee S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69(5):435–51. https://doi.org/10.1007/s00011-020-01318-0.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin Cd63 regulates Escrt-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21. https://doi.org/10.1016/j.devcel.2011.08.019.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
You X, Wang Y, Meng J, Han S, Liu L, Sun Y, et al. Exosomal Mir663b exposed to Tgfbeta1 promotes cervical cancer metastasis and epithelialmesenchymal transition by targeting Mgat3. Oncol Rep. 2021. https://doi.org/10.3892/or.2021.7963.
Mohammadi S, Yousefi F, Shabaninejad Z, Movahedpour A, Mahjoubin Tehran M, Shafiee A, et al. Exosomes and cancer: from oncogenic roles to therapeutic applications. IUBMB Life. 2020;72(4):724–48. https://doi.org/10.1002/iub.2182.
Xue D, Han J, Liang Z, Jia L, Liu Y, Tuo H, et al. Current perspectives on the unique roles of exosomes in drug resistance of hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:99–112. https://doi.org/10.2147/JHC.S351038.
Pusta A, Tertis M, Graur F, Cristea C, Al HN. Aptamers and new bioreceptors for the electrochemical detection of biomarkers expressed in hepatocellular carcinoma. Curr Med Chem. 2022. https://doi.org/10.2174/0929867329666220222113707.
Bastos N, Ruivo CF, da Silva S, Melo SA. Exosomes in cancer: use them or target them? Semin Cell Dev Biol. 2018;78:13–21. https://doi.org/10.1016/j.semcdb.2017.08.009.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044–61. https://doi.org/10.1016/j.cell.2020.07.009 (e18).
Marques P, Grossman AB, Korbonits M. The tumour microenvironment of pituitary neuroendocrine tumours. Front Neuroendocrinol. 2020;58:100852. https://doi.org/10.1016/j.yfrne.2020.100852.
Borriello L, Seeger RC, Asgharzadeh S, DeClerck YA. More than the genes, the tumor microenvironment in neuroblastoma. Cancer Lett. 2016;380(1):304–14. https://doi.org/10.1016/j.canlet.2015.11.017.
Konjevic GM, Vuletic AM, Mirjacic Martinovic KM, Larsen AK, Jurisic VB. The role of cytokines in the regulation of Nk cells in the tumor environment. Cytokine. 2019;117:30–40. https://doi.org/10.1016/j.cyto.2019.02.001.
Jacobs B, Ullrich E. The interaction of Nk cells and dendritic cells in the tumor environment: how to enforce Nk Cell & Dc action under immunosuppressive conditions? Curr Med Chem. 2012;19(12):1771–9. https://doi.org/10.2174/092986712800099857.
Russo E, Laffranchi M, Tomaipitinca L, Del Prete A, Santoni A, Sozzani S, et al. Nk Cell anti-tumor surveillance in a myeloid cell-shaped environment. Front Immunol. 2021;12:787116. https://doi.org/10.3389/fimmu.2021.787116.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. https://doi.org/10.1084/jem.183.3.1161.
Quah BJ, O’Neill HC. Mycoplasma contaminants present in exosome preparations induce polyclonal B cell responses. J Leukoc Biol. 2007;82(5):1070–82. https://doi.org/10.1189/jlb.0507277.
Mao Y, Wang Y, Dong L, Zhang Q, Wang C, Zhang Y, et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and Pd-1(High) Breg cells. Cancer Sci. 2019;110(9):2700–10. https://doi.org/10.1111/cas.14122.
Vallhov H, Gutzeit C, Johansson SM, Nagy N, Paul M, Li Q, et al. Exosomes containing glycoprotein 350 released by Ebv-transformed B cells selectively target b cells through Cd21 and Block Ebv infection in vitro. J Immunol. 2011;186(1):73–82. https://doi.org/10.4049/jimmunol.1001145.
Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T Cells (Treg). PLoS One. 2010. https://doi.org/10.1371/journal.pone.0011469.
Obermajer N, Urban J, Wieckowski E, Muthuswamy R, Ravindranathan R, Bartlett DL, et al. Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents. Nat Protoc. 2018;13(2):335–57. https://doi.org/10.1038/nprot.2017.130.
Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, et al. Breast cancer-derived exosomes alter macrophage polarization via Gp130/Stat3 signaling. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.00871.
Pekarev OG, Pekareva EO, Mayborodin IV, Silachev DN, Baranov II, Pozdnyakov IM, et al. The potential of extracellular microvesicles of mesenchymal stromal cells in obstetrics. J Matern Fetal Neonatal Med. 2021. https://doi.org/10.1080/14767058.2021.1951213.
Pistono C, Bister N, Stanova I, Malm T. Glia-derived extracellular vesicles: role in central nervous system communication in health and disease. Front Cell Dev Biol. 2020;8:623771. https://doi.org/10.3389/fcell.2020.623771.
Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, et al. Role of extracellular vesicles and micrornas on dysfunctional angiogenesis during preeclamptic pregnancies. Front Physiol. 2016;7:98. https://doi.org/10.3389/fphys.2016.00098.
Lorenc T, Chrzanowski J, Olejarz W. Current perspectives on clinical use of exosomes as a personalized contrast media and theranostics. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12113386.
Grimaldi A, Zarone MR, Irace C, Zappavigna S, Lombardi A, Kawasaki H, et al. Non-coding Rnas as a new dawn in tumor diagnosis. Semin Cell Dev Biol. 2018;78:37–50. https://doi.org/10.1016/j.semcdb.2017.07.035.
Ding Y, Li W, Wang K, Xu C, Hao M, Ding L. Perspectives of the application of liquid biopsy in colorectal cancer. Biomed Res Int. 2020;2020:6843180. https://doi.org/10.1155/2020/6843180.
Barger JF, Rahman MA, Jackson D, Acunzo M, Nana-Sinkam SP. Extracellular mirnas as biomarkers in cancer. Food Chem Toxicol. 2016;98:66–72. https://doi.org/10.1016/j.fct.2016.06.010.
Wu Z, Yang Z, Dai Y, Zhu Q, Chen LA. Update on liquid biopsy in clinical management of non-small cell lung cancer. Onco Targets Ther. 2019;12:5097–109. https://doi.org/10.2147/OTT.S203070.
Lan B, Zeng S, Grutzmann R, Pilarsky C. The role of exosomes in pancreatic cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20184332.
Visci G, Tolomeo D, Agostini A, Traversa D, Macchia G, Storlazzi CT. Circrnas and fusion-circrnas in cancer: new players in an old game. Cell Signal. 2020;75:109747. https://doi.org/10.1016/j.cellsig.2020.109747.
Hao X, Sun G, Zhang Y, Kong X, Rong D, Song J, et al. Targeting immune cells in the tumor microenvironment of Hcc: new opportunities and challenges. Front Cell Dev Biol. 2021;9:775462. https://doi.org/10.3389/fcell.2021.775462.
Bitzer M, Spahn S, Babaei S, Horger M, Singer S, Schulze-Osthoff K, et al. Targeting extracellular and juxtamembrane Fgfr2 mutations in chemotherapy-refractory cholangiocarcinoma. NPJ Precis Oncol. 2021;5(1):80. https://doi.org/10.1038/s41698-021-00220-0.
Guo CY, Liu JB, Zhou QB, Song JM, Zhang ZY, Li Z, et al. Exosomal noncoding Rnas and tumor drug resistance. Can Res. 2020;80(20):4307–13. https://doi.org/10.1158/0008-5472.Can-20-0032.
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–77. https://doi.org/10.1073/pnas.1521230113.
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated Kras and P53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–75. https://doi.org/10.1074/jbc.C113.532267.
Melzer C, Rehn V, Yang Y, Bahre H, von der Ohe J, Hass R. Taxol-loaded Msc-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11060798.
Shamili FH, Bayegi HR, Salmasi Z, Sadri K, Mahmoudi M, Kalantari M, et al. Exosomes derived from trail-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int J Pharm. 2018;549(1–2):218–29. https://doi.org/10.1016/j.ijpharm.2018.07.067.
Ran L, Tan X, Li Y, Zhang H, Ma R, Ji T, et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66. https://doi.org/10.1016/j.biomaterials.2016.02.025.
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. https://doi.org/10.1038/ncomms2282.
Chen J, Sun W, Zhang H, Ma J, Xu P, Yu Y, et al. Macrophages reprogrammed by lung cancer microparticles promote tumor development via release of Il-1beta. Cell Mol Immunol. 2020;17(12):1233–44. https://doi.org/10.1038/s41423-019-0313-2.
Cheow ES, Cheng WC, Lee CN, de Kleijn D, Sorokin V, Sze SK. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for myocardial ischemic (Mi) injury. Mol Cell Proteom. 2016;15(8):2628–40. https://doi.org/10.1074/mcp.M115.055731.
Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, et al. Boosting anti-Pd-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12(1):440. https://doi.org/10.1038/s41467-020-20723-x.
Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4(7):743–53. https://doi.org/10.1038/s41551-020-0583-0.
Zhang W, Yu ZL, Wu M, Ren JG, Xia HF, Sa GL, et al. Magnetic and folate functionalization enables rapid isolation and enhanced tumor-targeting of cell-derived microvesicles. ACS Nano. 2017;11(1):277–90. https://doi.org/10.1021/acsnano.6b05630.
Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78(3):798–808. https://doi.org/10.1158/0008-5472.CAN-17-2880.
Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. https://doi.org/10.1016/j.biomaterials.2020.120467.
Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki JI, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018;563(7733):719–23. https://doi.org/10.1038/s41586-018-0729-3.
Sadik CD, Miyabe Y, Sezin T, Luster AD. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin Immunol. 2018;37:21–9. https://doi.org/10.1016/j.smim.2018.03.002.
Zhao K, Zhang Y, Zhang X, Li W, Shi C, Guo C, et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in chitosan nanoparticles. Int J Nanomed. 2014;9:389–402. https://doi.org/10.2147/IJN.S54226.
Zhao K, Li W, Huang T, Luo X, Chen G, Zhang Y, et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in Plga nanoparticles. PLoS One. 2013;8(12):e82648. https://doi.org/10.1371/journal.pone.0082648.
Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8(10):1581–8. https://doi.org/10.1586/14737140.8.10.1581.
Munguia A, Ota T, Miest T, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15(10):797–806. https://doi.org/10.1038/gt.2008.45.
Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629. https://doi.org/10.1155/2012/805629.
Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg MW, et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci USA. 2017;114(30):E6157–65. https://doi.org/10.1073/pnas.1700363114.
Zhao K, Zhang Y, Zhang X, Shi C, Wang X, Wang X, et al. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int J Nanomed. 2014;9:4609–19. https://doi.org/10.2147/IJN.S70633.
Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via Cgas/sting signaling. Cancer Immunol Res. 2015;3(2):196–205. https://doi.org/10.1158/2326-6066.CIR-14-0177.
Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–9. https://doi.org/10.1016/s1470-2045(00)00166-2.
Lim CK, Heo J, Shin S, Jeong K, Seo YH, Jang WD, et al. Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Lett. 2013;334(2):176–87. https://doi.org/10.1016/j.canlet.2012.09.012.
Pinto A, Marangon I, Mereaux J, Nicolas-Boluda A, Lavieu G, Wilhelm C, et al. Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 2021;15(2):3251–63. https://doi.org/10.1021/acsnano.0c09938.
Yang Z, Wang J, Ai S, Sun J, Mai X, Guan W. Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging. Theranostics. 2019;9(23):6809–23. https://doi.org/10.7150/thno.36988.
Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47(16):3897–915. https://doi.org/10.1021/jm040074b.
Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074. https://doi.org/10.1038/s41467-018-03473-9.
Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26(11):612–21. https://doi.org/10.1016/j.tibtech.2008.07.007.
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029.
Li SY, Cheng H, Xie BR, Qiu WX, Zeng JY, Li CX, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–18. https://doi.org/10.1021/acsnano.7b02533.
Liu L, Zhou X, Zheng R, Huang J, Kong R, Li Y, et al. Self-delivery nanomedicine for chemotherapy sensitized photodynamic therapy. Chem Commun (Camb). 2021;57(59):7296–9. https://doi.org/10.1039/d1cc02318g.
Wang Y, Xie Y, Li J, Peng ZH, Sheinin Y, Zhou J, et al. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano. 2017;11(2):2227–38. https://doi.org/10.1021/acsnano.6b08731.
Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3(5):558–61. https://doi.org/10.1038/nm0597-558.
Yang X, Xie Y. Recent advances in polymeric core-shell nanocarriers for targeted delivery of chemotherapeutic drugs. Int J Pharm. 2021;608:121094. https://doi.org/10.1016/j.ijpharm.2021.121094.
He J, Zheng R, Zhang Z, Tan J, Zhou C, Zhang G, et al. Collagen I enhances the efficiency and anti-tumor activity of dendritic-tumor fusion cells. Oncoimmunology. 2017;6(12):e1361094. https://doi.org/10.1080/2162402X.2017.1361094.
Lu S, Yang N, He J, Gong W, Lai Z, Xie L, et al. Generation of cancer-specific cytotoxic Pd-1(-) T cells using liposome-encapsulated Crispr/cas system with dendritic/tumor fusion cells. J Biomed Nanotechnol. 2019;15(3):593–601. https://doi.org/10.1166/jbn.2019.2712.
Acknowledgements
Thank all authors.
Funding
There is no fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Xiang, Y., Yang, Y. et al. The application of tumor cell-derived vesicles in oncology therapy. Clin Transl Oncol 25, 364–374 (2023). https://doi.org/10.1007/s12094-022-02966-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02966-w