Abstract
Background
Previous studies have shown that the ability of tumor cells to move and migrate is related to the molecular chain pathway mediated by actin. This study focused on the molecular mechanism of gelsolin (GSN) as an important actin-binding protein in promoting HCC invasion and metastasis.
Methods
The relationship between GSN expression and clinical characteristics was observed by immunohistochemistry (IHC). In vitro and in vivo experiments confirmed the role of GSN in HCC metastasis. Dual-immunoprecipitation (IP), immunofluorescence (IF), western blotting, and the gelatinase activity assay were used to investigate the mechanism of GSN-promoting metastasis. PEX fusion proteins were used to intervene in the transfer molecular chain.
Results
Our study found that GSN promoted HCC invasion and metastasis through its synergistic effect with actin-related transfer molecular chain (actin-CD44-MMPs). Concretely, as an important binding molecule of actin, GSN activated MMP2 by interacting with MMP14. Furthermore, CD44 might be a key node in the above-mentioned mechanism. The use of MMP14 domain (PEX fusion protein) to competitively bind to CD44 helped to inhibit the activation of downstream MMP2.
Conclusions
GSN played crucial roles in HCC metastatic process. An improved understanding of the multiple effects of GSN in HCC might facilitate a deeper appreciation of GSN as an important HCC regulator. The study identified GSN and its regulated transfer molecular chain as potential therapeutic targets for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HCC is one of the most common and aggressive malignancies and the third leading cause of cancer-related deaths [1]. The better understanding of the cellular and molecular mechanisms of HCC will help us to find more advanced treatments. Recently, the influence of cytoskeletal changes in the migration ability of tumor cells is being concerned. GSN, which is a Ca2+-dependent actin filament severing and capping protein, has been identified as an important player in tumorigenesis. Abnormal expression of GSN has been observed in many types of cancers [2,3,4,5]. The alteration of GSN results in altered actin cytoskeletal architecture and increased cell motility, both of which are essential for tumor cellular migration and invasive growth.
Our results revealed a significant positive correlation between increased GSN and HCC metastatic behavior. It has been reported that GSN could be a biomarker for evaluating the lymph-node metastasis of HCC [6]. Some researchers also considered GSN continuously up-regulated during the entire invasion process [7], promoted HCC progression by regulating epithelial–mesenchymal transition, and correlated with a poor prognosis [8]. Although the above evidence implied a potential role of GSN as a metastasis-associated molecule in HCC, its molecular regulation in the invasion process was still unknown. In this study, we aimed to investigate the mechanism by which GSN acts as an actin-binding protein to promote HCC metastasis.
Methodology
Patients and specimens
Tissue samples of HCC and para-carcinoma were obtained from the Affiliated Tumor Hospital of Guangxi Medical University (Nanning, China). The final paraffin-embedded tissue samples were derived from 69 HCC patients.
Cell culture and recombinant plasmid DNA
The HCC cell line HepG2 was purchased from the Institute of Biochemistry and Cell Biology, Shanghai, China. Expressed or shRNA fractions were connected to pCDH–CMV-MCS-EF1-Puro or pGFP-C-shLenti vectors, respectively. Over-expressed pCDH-GSN, pCDH-MMP14 or inhibited GFP-GSN, GFP-MMP14 plasmids were transfected into HepG2 cells. pCDH-Control and GFP-Control were transfected as contrast.
IHC
Three consecutive slides of each tissue block were prepared to replicate the experiments. IHC was performed according to the protocol of antibody (Abcam plc, Cambridge, UK). Negative control slides omitting the primary antibodies were included in the assay.
Wound-healing assay
The experiment was performed by plating 3.5 × 105 cells in 6-well plates and carried out in triplicate. Photographs of cells invading the scratch were taken at the indicated time points.
Migration and invasion assay
Cell culture inserts were seeded with 2.5 × 104 cells. The experiments were carried out in triplicate according to the instruction of BD BioCoat™ Matrigel™ kit (Corning, NY, USA).
Nude mice model of metastasis
A total of 1 × 106 transfected HepG2 cells were implanted into the caudal vein of 6-week-old male BALB/C allogeneic athymic nude mice. Eight mice were used in each group. The mice were killed and dissected 27 days after tumor inoculation. The metastasis incidence and tumor numbers were counted. Paraffin sections and HE staining were prepared to follow the routine procedures to verify tumorigenesis pathologically.
Dual-IP
Dual-IP was performed according to the instruction of Flag-HA double tag IP kits (Thermo Fisher Scientific, NY, USA). We extra employed HepG2 cells that were transfected with the pCDH-GSN-FLAG-HA construct. HepG2 cells that expressed pCDH-HA-FLAG without a GSN fragment were served as a control.
Western blotting
Protein samples were prepared according to the instructions of Mammalian Protein Extraction Kit, Membrane Protein Extraction Kit, and Cytoplasmic Extraction Kit (Thermo Fisher Scientific Co., Ltd, Shanghai, China). Western blotting was performed according to the previous method of our research group [9].
IF
We extra prepared HepG2 cells that were co-transfected with the pCDH-GSN and pCDH-MMP14 plasmids (GSN/MMP14). A total of 5 × 106 transfected HepG2 cells were planted on the glass slide. Prepared slides were mounted for imaging using a confocal system (Nikon, Tokyo, Japan).
Non-denaturing electrophoresis of gelatin substrates
The experiment was carried out according to the instructions of Gelatin Zymography Kit (Zhongkeritai Biotechnology Co., Ltd, Beijing, China).
In-cell western blotting
A total of 5 × 106 transfected cells were inoculated on 96-well plates. The expression of target protein was calculated as the ratio of target protein to GAPDH. The experiment was conducted in three replicates.
Statistical analysis
SPSS19.0 was used for statistical analysis, and the results were expressed as mean ± SD. Independent sample t test was used for comparison between the two groups. Kruskal–Wallis test was used to compare multiple continuous independent samples. Rank sum test was used for pathological change grade data. P < 0.05 was considered significant.
Results
GSN promotes HCC invasion and metastasis
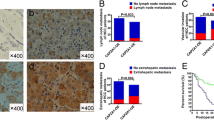
The clinical characteristics of 69 HCC patients are listed in Table 1. Statistical analysis revealed that GSN expression was higher in patients with PVTT (portal vein tumor thrombus) and metastasis. Patients with HCC metastasis had more positive expression rates (88.9%) than those with non-metastasis (43.2%), **P < 0.01 (Fig. 1A). However, there were no significant differences between GSN expression and the patients’ gender, age, tumor numbers, tumor size, or recurrence. Wound-healing assay showed that GSN promoted cells to move and heal 84.2% of wounded areas while comparing to the shGSN cells with only a 40.4% healing rate, *P < 0.05. In migration and invasion assay, pCDH-GSN group also showed a number of 278 ± 11 migrating cells, which was significantly higher than that of the GFP-GSN (72 ± 5), *P < 0.05 (Fig. 1B). The results of in vivo experiment showed that 75% of the mice which transplanted with pCDH-GSN developed severe lung metastasis. The number of metastasis tumors was also much higher in pCDH-GSN group when compared with the control, *P < 0.05 (Fig. 1C).
The relationship between GSN expression and HCC metastasis. A Expression intensity of positively staining cells: staining cells were divided into different grades (−, + , ++, and +++), *P < 0.05, **P < 0.01. B In vitro invasion and metastasis assays, *P < 0.05. C Lung gross observation: the white arrows were used to point out the metastatic tumor, *P < 0.05
GSN interacts with MMP14
Dual-IP suggested that GSN interacted with transfer factor MT-MMP and MMP14 (Table 2). Interestingly, MMP14 belongs to the membrane MMP (MT-MMP) subfamily, and each member of this subfamily contains a potential transmembrane domain, suggesting that MMP14 functions at the cell membrane. It has been reported that MMP14 can activate other members of the MMP family, such as MMP2 [10]. Therefore, we tried to examine if GSN level altered the expression of MMP14 and MMP2. As shown in Fig. 2A, MMP14 and MMP2 were up-regulated in pCDH-GSN cells, *P < 0.05. Then we constructed GSN and MMP14 co-expressed cells (GSN/MMP14) to observe the interaction between GSN and MMP14. We extracted the cytoplasmic proteins and membrane proteins of GSN/MMP14, pCDH-MMP14, pCDH-GSN, and pCDH-Control, respectively, and analyzed the expression of GSN and MMP14 in cytoplasm and membrane. The results showed that GSN was expressed in the cytoplasm rather than cell membrane. When GSN was up-regulated in the cytoplasm, the expression of MMP14 also increased while comparing to the control cells. However, when GSN and MMP14 were co-expressed, the expression of MMP14 was mainly up-regulated in cell membrane (Fig. 2B). Through IF stain (Fig. 2C), we found that MMP14 was not only increased, but also strongly expressed on the cellular surface of the co-expressed cells. The above results suggested that GSN interacted with MMP14 and induced the translocation of MMP14 from cytoplasm to membrane.
Interaction between GSN and MMP14. A The relationship between GSN level and the expression of MMP14 and MMP2, *P < 0.05. B The expression and co-expression of GSN, MMP14 in cytoplasm and cell membrane. C Subcellular localization of GSN, MMP14 expression and co-expression: the red arrows were used to point out the expression of MMP14 on the cell membrane
GSN promotes HCC metastasis through actin-related transfer molecular chain
We transfected MMP14 shRNA plasmid into pCDH-GSN cells to explore whether MMP14 affects the metastasis function of GSN. Wound-healing assay showed that GSN promoted cells to move and heal 57.4% of wounded areas. However, with MMP14 knocking down, the healing rate was only 10.3%, *P < 0.05. Meanwhile, when MMP14 was down-regulated, the motility and invasiveness of HCC cells were also significantly decreased, *P < 0.05 (Fig. 3A). The possible mechanism pointed to the decreased activity of MMPs (especially MMP2), *P < 0.05 (Fig. 3B).
Previous research reported that the biological process of tumor metastasis depends on the adhesion molecule. Then, we noticed the reports about CD44 (a linker of actin), which involves in actin skeleton remodeling, regulates the cell adhesion and movement, and is directly related to the cell invasion and migration [11, 12]. It has been found that the PEX domain of MMP14 can bind to CD44 on the cell surface, resulting in intracellular cytoskeletal rearrangement as well as cellular migration and invasion, through the activation of MMP2 [13]. Therefore, we tried to explore the relationship between GSN level and the expression of MMP2 and CD44. The results showed that CD44 and MMP2 were up-regulated in GSN over-expressed cells, *P < 0.05, **P < 0.01 (Fig. 3C), which revealed a potential mechanism for GSN promotes HCC metastasis through actin-related transfer molecular chain (atin-CD44-MMPs).
PEX fusion protein blocks the transfer molecular chain
After exploring IC50 by cytotoxicity assay, 30 mM/vehicle PEX fusion protein was acted on pCDH-GSN cells (PEX-GSN). The results showed that PEX inhibited the activity of MMP2 (Fig. 4A). While using cisplatin and 5, fluorouracil as positive controls to analyze the effect of PEX in intervening the transfer molecular chain, the results showed that PEX fusion protein inhibited the metastasis ability of GSN over-expressed cells, *P < 0.05 (Fig. 4B).
Discussion
GSN is ubiquitously expressed in different cell types and functions as a multifunctional regulator of cell [14]. Here, we focused on its impacts on HCC metastasis. An improved understanding of the function and regulatory mechanisms of GSN may lead to new considerations of this protein as a metastasis regulatory molecule for HCC.
In this study, we revealed the relationship between GSN expression and HCC metastasis. Then, we demonstrated the role of GSN in promoting the invasion and metastasis of HCC and identified a possible mechanism related to transfer molecular chain (actin-CD44-MMPs). Previous studies have found that the movement, migration, adhesion, and invasion behavior of tumor cells are related to actin [15, 16]. Our result suggested that the changes between actin cytoskeleton and extracellular matrix proteins induced to HCC metastasis. This process could be affected by many factors, depended on adhesion molecules, and might be regulated by specific actin-binding factors such as GSN.
We confirmed the interaction between GSN and MMP14. Increased expression of MMP14 in tumor cells enhances the growth, invasion, and metastasis of tumor [17]. MMP14 is generally considered pro-invasive and pro-tumorigenic, because: (1) the expression and activity of MMP14 is elevated in tumor cells and (2) high levels of MMP14 directly correlate with enhanced cell migration. (3) MMP14 can activate other MMP family members [18, 19]. In this paper, we speculated that MMP14 and MMP2 might be the terminal molecules in GSN-mediated transfer mechanism.
CD44, a member of the adhesion molecule family, has been proved to be a linker of actin [20]. Literature has shown that CD44 is related to the tumor metastasis caused by changes in actin cytoskeleton [21, 22]. Furthermore, CD44 could specifically interact with the PEX sequence of MMP14, activate MMP2, and regulate cell migration, suggesting that CD44 and MMP2 may be closely related to the tumor metastasis. Here, we considered that CD44 might be a key node in actin-related transfer molecular chain and act together with actin and MMP family members on the biological processes of HCC metastasis.
Conclusion
In conclusion, we found that GSN played crucial roles in HCC metastatic processes. Earlier studies of GSN that focused on HCC metastasis are now being extended to mechanisms by which GSN is involved in metastatic behavior. This study identified GSN and its regulated actin transfer molecular chain as potential therapeutic targets for HCC.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- GSN:
-
Gelsolin
- IHC:
-
Immunohistochemistry
- IP:
-
Immunoprecipitation
- IF:
-
Immunofluorescence
- MMP:
-
Matrix metalloproteinase
- ICC:
-
Immunocytochemistry
- PVTT:
-
Portal vein tumor thrombus
- MT-MMP:
-
Membrane-type 1 matrix metalloproteinase
References
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021. https://doi.org/10.1002/hep.31288.
Wu Y, Zheng J, Yan Y, Liu J, Zhou Y. Gelsolin can be a prognostic biomarker and correlated with immune infiltrates in gastric cancer. Int J Gen Med. 2022;15:927–36. https://doi.org/10.2147/IJGM.S339940.
Oelrich F, Junker H, Stope MB, Erb HHH, Walther R, Venz S, et al. Gelsolin governs the neuroendocrine transdifferentiation of prostate cancer cells and suppresses the apoptotic machinery. Anticancer Res. 2021;41(8):3717–29. https://doi.org/10.21873/anticanres.15163.
Lee HJ, Kim MJ, Kim YS, Choi MY, Cho GJ, Choi WS. UHRF1 silences gelsolin to inhibit cell death in early stage cervical cancer. Biochem Biophys Res Commun. 2020;526(4):1061–8. https://doi.org/10.1016/j.bbrc.2020.03.185.
Bahrami S, Gheysarzadeh A, Sotoudeh M, Bandehpour M, Khabazian R, Zali H, et al. The association between gelsolin-like actin-capping protein (CapG) overexpression and bladder cancer prognosis. Urol J. 2020;18(2):186–93. https://doi.org/10.22037/uj.v0i0.5664.
Qazi AS, Sun M, Huang Y, Wei Y, Tang J. Subcellular proteomics: determination of specific location and expression levels of lymphatic metastasis associated proteins in hepatocellular carcinoma by subcellular fractionation. Biomed Pharmacother. 2011;65(6):407–16. https://doi.org/10.1016/j.biopha.2011.04.028.
Chen RX, Song HY, Dong YY, Hu C, Zheng QD, Xue TC, et al. Dynamic expression patterns of differential proteins during early invasion of hepatocellular carcinoma. PLoS ONE. 2014;9(3): e88543. https://doi.org/10.1371/journal.pone.0088543.
Zhang Y, Luo X, Lin J, Fu S, Feng P, Su H, et al. Gelsolin promotes cancer progression by regulating epithelial–mesenchymal transition in hepatocellular carcinoma and correlates with a poor prognosis. J Oncol. 2020;2020:1980368. https://doi.org/10.1155/2020/1980368.
Zhou Y, Deng X, Zang N, Li H, Li G, Li C, et al. Transcriptomic and proteomic investigation of HSP90A as a potential biomarker for HCC. Med Sci Monit. 2015;21:4039–49. https://doi.org/10.12659/MSM.896712.
Henderson N, Markwick LJ, Elshaw SR, Freyer AM, Knox AJ, Johnson SR. Collagen I and thrombin activate MMP-2 by MMP-14-dependent and-independent pathways: implications for airway smooth muscle migration. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L1030–8. https://doi.org/10.1152/ajplung.00317.2006.
Jensen PV, Larsson L-I. Actin microdomains on endothelial cells: association with CD44, ERM proteins, and signaling molecules during quiescence and wound healing. Histochem Cell Biol. 2004;121(5):361–9. https://doi.org/10.1007/s00418-004-0648-2.
Kahsai AW, Zhu S, Wardrop DJ, Lane WS, Fenteany G. Quinocarmycin analog DX-52-1 inhibits cell migration and targets radixin, disrupting interactions of radixin with actin and CD44. Chem Biol. 2006;13(9):973–83. https://doi.org/10.1016/j.chembiol.2006.07.011.
Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, et al. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biol Chem. 2011;286(38):33167–77. https://doi.org/10.1074/jbc.M111.256644.
Hsieh CH, Wang YC. Emerging roles of plasma gelsolin in tumorigenesis and modulating the tumor microenvironment. Kaohsiung J Med Sci. 2022;38(9):819–25. https://doi.org/10.1002/kjm2.12578.
Wehrle-Haller B, Imhof BA. Actin, microtubules and focal adhesion dynamics during cell migration. Int J Biochem Cell Biol. 2003;35(1):39–50. https://doi.org/10.1016/S1357-2725(02)00071-7.
Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692(2–3):159–74. https://doi.org/10.1016/j.bbamcr.2004.03.005.
Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, et al. The role of matrix metalloproteinases in the epithelial–mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol (Amst). 2019;2019:9423907. https://doi.org/10.1155/2019/9423907.
Yosef G, Arkadash V, Papo N. Targeting the MMP-14/MMP-2/integrin αvβ3 axis with multispecific N-TIMP2-based antagonists for cancer therapy. J Biol Chem. 2018;293(34):13310–26. https://doi.org/10.1074/jbc.RA118.004406.
Li Z, Takino T, Endo Y, Sato H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017;108(3):347–53. https://doi.org/10.1111/cas.13134.
Chabadel A, Bañon-Rodríguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, et al. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell. 2007;18(12):4899–910. https://doi.org/10.1091/mbc.e07-04-0378.
Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LYW. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope. 2009;119(8):1518–30. https://doi.org/10.1002/lary.20506.
Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114:5236–44. https://doi.org/10.1182/blood-2009-04-219204.
Acknowledgements
We thank Shaoshi Luo for technical assistance in animal experiments.
Funding
This work was supported by Natural Science Foundation of Guangxi Province (Grant No. 2018GXNSFAA050036).
Author information
Authors and Affiliations
Contributions
ZY and MH have contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was conducted in line with the Declaration of Helsinki. The ethical review board in Guangxi Medical University approved the study (ethical review document number 20200075).
Consent for publication
This article has been read and approved in the present form for submission by all authors.
Informed consent
Written informed consent to participate was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., He, M. GSN synergies with actin-related transfer molecular chain to promote invasion and metastasis of HCC. Clin Transl Oncol 25, 482–490 (2023). https://doi.org/10.1007/s12094-022-02961-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02961-1