Abstract
Background
Psychological distress and cognitive impairment are highly prevalent among patients with brain metastases after whole-brain radiotherapy (WBRT). Our purpose was to evaluate the correlations between psychological distress, cognitive impairment and quality of life in patients with brain metastases after WBRT.
Methods
Seventy-one patients with brain metastasis treated with WBRT were enrolled in this study and were investigated with several scales, including the Montreal Cognitive Assessment Scale (MoCA), the Functional Assessment of Cancer Therapy-Cognitive Function version 3 (FACT-Cog, version 3), the Functional Assessment of Cancer Therapy-Brain Module version 4 (FACT-Br, version 4) and the Psychological Distress Thermometer (DT), before and after WBRT.
Results
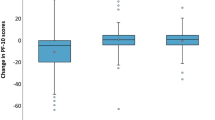
The MoCA, FACT-Cog and FACT-Br scores in patients with brain metastases were significantly decreased after WBRT compared with before WBRT (z = − 7.106, − 6.933 and − 6.250, respectively, P < 0.001), while the DT scores were significantly increased (z = 6.613, P < 0.001). There was an obvious negative correlation between the DT score and the FACT-Cog score (r = − 0.660, P < 0.001), a significant negative correlation between the DT score and the FACT-Br score (r = − 0.833, P < 0.001), and an obvious positive correlation between the FACT-Cog score and the FACT-Br score (r = 0.603, P < 0.001). These results suggest that WBRT can cause cognitive impairment in patients with brain metastases, increase their psychological distress and reduce their quality of life (QOL).
Conclusion
After receiving WBRT, the cognitive function and QOL of patients with brain metastases were decreased, while psychological distress increased. The cognitive impairment and the decline of QOL after WBRT are associated with increased psychological distress, and that the decline of QOL is associated with cognitive impairment of patients.






Similar content being viewed by others
Data availability
The data that support the study may be available upon request with permission from the researchers who collected the data.
Abbreviations
- DT:
-
Distress thermometer
- IMRT:
-
Intensity-modulated radiotherapy
- MoCA:
-
Montreal cognitive assessment scale
- QOL:
-
Quality of life
- SD:
-
Standard deviation
- SRS:
-
Stereotactic radiosurgery
- WBRT:
-
Whole-brain radiotherapy
References
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85.
Gagliano A, Prestifilippo A, Cantale O, Ferini G, Fisichella G, Fontana P, Sciacca D, Giuffrida D. Role of the combination of cyclin-dependent kinase inhibitors (cdki) and radiotherapy (rt) in the treatment of metastatic breast cancer (mbc): advantages and risks in clinical practice. Front Oncol. 2021;11: 643155.
Brunasso L, Ferini G, Bonosi L, Costanzo R, Musso S, Benigno UE, Gerardi RM, Giammalva GR, Paolini F, Umana GE, Graziano F, Scalia G, Sturiale CL, Di Bonaventura R, Iacopino DG, Maugeri R. A Spotlight on the role of radiomics and machine-learning applications in the management of intracranial meningiomas: a new perspective in neuro-oncology: a review. Life (Basel). 2022;12:4.
Wilke C, Grosshans D, Duman J, Brown P, Li J. Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol. 2018;20:597–607.
Giammalva GR, Ferini G, Musso S, Salvaggio G, Pino MA, Gerardi RM, Brunasso L, Costanzo R, Paolini F, Di Bonaventura R, Umana GE, Graziano F, Palmisciano P, Scalia G, Tumbiolo S, Midiri M, Iacopino DG, Maugeri R. Intraoperative ultrasound: emerging technology and novel applications in brain tumor surgery. Front Oncol. 2022;12: 818446.
Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, Diefenbach CSM, Rodriguez-Galindo C, George DJ, Gilligan TD, Harvey RD, Johnson ML, Kimple RJ, Knoll MA, LoConte N, Maki RG, Meisel JL, Meyerhardt JA, Pennell NA, Rocque GB, Sabel MS, Schilsky RL, Schneider BJ, Tap WD, Uzzo RG, Westin SN. Clinical cancer advances 2020: annual report on progress against cancer from the American Society Of Clinical Oncology. J Clin Oncol. 2020;38:1081.
Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Chang SD. Brain metastases. Nat Rev Dis Primers. 2019;5:5.
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB, Bahary JP, Hadjipanayis CG, Urbanic JJ, Barker FG 2nd, Farace E, Khuntia D, Giannini C, Buckner JC, Galanis E, Roberge D. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–60.
Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13:52–64.
Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–300.
Stereotactic Radiosurgery Keeps Brain Metastases at Bay. Cancer Discov. 2016 Nov;6(11):OF2. doi:10.1158/2159-8290.CD-NB2016-124. Epub 2016 Oct 7. PMID: 27807104.
Grosshans DR, Mohan R, Gondi V, Shih HA, Mahajan A, Brown PD. The role of image-guided intensity modulated proton therapy in glioma. Neuro Oncol. 2017;19:ii30–7.
Lang-Rollin I, Berberich G. Psycho-oncology. Dialogues Clin Neurosci. 2018;20:13–22.
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, Li X, Li Y, Xiong W, Li G, Guo C, Zeng Z. Chronic stress promotes cancer development. Front Oncol. 2020;10:1492.
Steineck A, Bradford MC, Lau N, Scott S, Yi-Frazier JP, Rosenberg AR. A psychosocial intervention’s impact on quality of life in ayas with cancer: a post hoc analysis from the promoting resilience in stress management (prism) randomized controlled trial. Children (Basel). 2019;6:11.
Compen F, Bisseling E, Schellekens M, Donders R, Carlson L, van der Lee M, Speckens A. Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36:2413–21.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Tong T, Lu H, Zong J, Lv Q, Chu X. Chemotherapy-related cognitive impairment in patients with breast cancer based on MRS and DTI analysis. Breast Cancer. 2020;27:893–902.
Wei X, Yuan R, Yang J, Zheng W, Jin Y, Wang M, Jiang J, Wu C, Li K. Effects of Baduanjin exercise on cognitive function and cancer-related symptoms in women with breast cancer receiving chemotherapy: a randomized controlled trial. Support Care Cancer. 2022;30:6079–91.
Zhao H, Li X, Zhou C, Wu Y, Li W, Chen L. Psychological distress among Chinese patients with breast cancer undergoing chemotherapy: concordance between patient and family caregiver reports. J Adv Nurs. 2022;78:750–64.
Chiu N, Chiu L, Zeng L, Zhang L, Cella D, Popovic M, Chow R, Lam H, Poon M, Chow E. Quality of life in patients with primary and metastatic brain tumors in the literature as assessed by the FACT-Br. World J Oncol. 2012;3:280–5.
Wang Y, Zou L, Jiang M, Wei Y, Jiang Y. Measurement of distress in Chinese inpatients with lymphoma. Psychooncology. 2013;22:1581–6.
Decat BC, de Araujo CFTC. Assessment of distress and quality of life of cancer patients over the course of chemotherapy. Invest Educ Enferm. 2014;32:216–24.
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–74.
Liu F, Huang J, Zhang L, Fan F, Chen J, Xia K, Liu Z. Screening for distress in patients with primary brain tumor using distress thermometer: a systematic review and meta-analysis. BMC Cancer. 2018;18:124.
Ninu MB, Miccinesi G, Bulli F, De Massimi A, Muraca MG, Franchi G, Squadrelli SM. Psychological distress and health-related quality of life among head and neck cancer patients during the first year after treatment. Tumori. 2016;102:96–102.
Nicol C, Ownsworth T, Cubis L, Nguyen W, Foote M, Pinkham MB. Subjective cognitive functioning and associations with psychological distress in adult brain tumour survivors. J Cancer Surviv. 2019;13:653–62.
Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860–71.
Connor M, Karunamuni R, McDonald C, White N, Pettersson N, Moiseenko V, Seibert T, Marshall D, Cervino L, Bartsch H, Kuperman J, Murzin V, Krishnan A, Farid N, Dale A, Hattangadi-Gluth J. Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2016;121:209–16.
Kayama T, Sato S, Sakurada K, Mizusawa J, Nishikawa R, Narita Y, Sumi M, Miyakita Y, Kumabe T, Sonoda Y, Arakawa Y, Miyamoto S, Beppu T, Sugiyama K, Nakamura H, Nagane M, Nakasu Y, Hashimoto N, Terasaki M, Matsumura A, Ishikawa E, Wakabayashi T, Iwadate Y, Ohue S, Kobayashi H, Kinoshita M, Asano K, Mukasa A, Tanaka K, Asai A, Nakamura H, Abe T, Muragaki Y, Iwasaki K, Aoki T, Watanabe T, Sasaki H, Izumoto S, Mizoguchi M, Matsuo T, Takeshima H, Hayashi M, Jokura H, Mizowaki T, Shimizu E, Shirato H, Tago M, Katayama H, Fukuda H, Shibui S. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority randomized controlled trial. J Clin Oncol. 2018;36:3282.
Ferini G, Viola A, Valenti V, Tripoli A, Molino L, Marchese VA, Illari SI, Rita Borzì G, Prestifilippo A, Umana GE, Martorana E, Mortellaro G, Ferrera G, Cacciola A, Lillo S, Pontoriero A, Pergolizzi S, Parisi S. Whole brain irradiation or stereotactic radiosurgery for five or more brain metastases (WHOBI-STER): a prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin Transl Radiat Oncol. 2022;32:52–8.
Yoo DH, Song SW, Yun TJ, Kim TM, Lee SH, Kim JH, Sohn CH, Park SH, Park CK, Kim IH, Choi SH. MR imaging evaluation of intracerebral hemorrhages and T2 hyperintense white matter lesions appearing after radiation therapy in adult patients with primary brain tumors. PLoS ONE. 2015;10:e0136795.
Sindoni A, Severo C, Vadala RE, Ferini G, Mazzei MM, Vaccaro M, IatÌ G, Pontoriero A, Pergolizzi S. Levetiracetam-induced radiation recall dermatitis in a patient undergoing stereotactic radiotherapy. J Dermatol. 2016;43:1440–1.
Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, Hampson RE, Deadwyler SA, Cline JM. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017;187:599–611.
Davis CM, Roma PG, Armour E, Gooden VL, Brady JV, Weed MR, Hienz RD. Effects of X-ray radiation on complex visual discrimination learning and social recognition memory in rats. PLoS ONE. 2014;9: e104393.
Baddour AAD, Apodaca LA, Alikhani L, Lu C, Minasyan H, Batra RS, Acharya MM, Baulch JE. Sex-specific effects of a wartime-like radiation exposure on cognitive function. Radiat Res. 2020;193:5–15.
Betlazar C, Middleton RJ, Banati RB, Liu GJ. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016;9:144–56.
Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–6.
Reygagne E, Du Boisgueheneuc F, Berger A. Brain metastases: Focal treatment (surgery and radiation therapy) and cognitive consequences. Bull Cancer. 2017;104:344–55.
Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, Rakovitch E, Chow E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;1:Cd003869.
Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. 2016;45:139–62.
Sahgal A, Ruschin M, Ma L, Verbakel W, Larson D, Brown PD. Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro Oncol. 2017;19:ii5–15.
Jing Z, Li J, Wang Y, Ding L, Tang X, Feng Y, Zhou C. The mediating effect of psychological distress on cognitive function and physical frailty among the elderly: evidence from rural Shandong China. J Affect Disord. 2020;268:88–94.
Fan XW, Chen F, Chen Y, Chen GH, Liu HH, Guan SK, Deng Y, Liu Y, Zhang SJ, Peng WJ, Jiang GL, Wu KL. Electroacupuncture prevents cognitive impairments by regulating the early changes after brain irradiation in rats. PLoS ONE. 2015;10:e0122087.
Cacao E, Cucinotta FA. Modeling impaired hippocampal neurogenesis after radiation exposure. Radiat Res. 2016;185:319–31.
Yang WC, Chen YF, Yang CC, Wu PF, Chan HM, Chen JL, Chen GY, Cheng JC, Kuo SH, Hsu FM. Hippocampal avoidance whole-brain radiotherapy without memantine in preserving neurocognitive function for brain metastases: a phase II blinded randomized trial. Neuro Oncol. 2021;23:478–86.
Robin TP, Rusthoven CG. Strategies to preserve cognition in patients with brain metastases: a review. Front Oncol. 2018;8:415.
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–9.
Shi S, Sandhu N, Jin MC, Wang E, Jaoude JA, Schofield K, Zhang C, Liu E, Gibbs IC, Hancock SL, Chang SD, Li G, Hayden-Gephart M, Adler JR, Soltys SG, Pollom EL. Stereotactic radiosurgery for resected brain metastases: single-institutional experience of over 500 cavities. Int J Radiat Oncol Biol Phys. 2020;106:764–71.
Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Bovi JA, Robinson C, Konski A, Khuntia D, Grosshans D, Benzinger TLS, Bruner D, Gilbert MR, Roberge D, Kundapur V, Devisetty K, Shah S, Usuki K, Anderson BM, Stea B, Yoon H, Li J, Laack NN, Kruser TJ, Chmura SJ, Shi W, Deshmukh S, Mehta MP, Kachnic LA. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38:1019–29.
Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18:21.
Acknowledgements
Thanks to the cancer patients who are confronting with cancer. Their dedication and persistence have promoted the advance of the anti-cancer career. We sincerely wish these cancer survivors restore their mental and physical health. We would like to thank the reviewers for their valuable comments.
Funding
Funding was supplied by National Natural Science Foundation of China (No. 81872504; 81372487). Funders were not involved in the formulation and completion of the research.
Author information
Authors and Affiliations
Contributions
Conceptualization: HC, SY, HZ. Data collection: HZ, SY, WL. Data analysis: SY, HZ. Original draft writing: HZ, SY. Review and editing: SY, YC, QZ, WL, LP, YJ, XY. SY, HZ, WL contributed equally to this work. And the publication of this manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University, and all included patients provided a written informed consent.
Informed consent
All included patients provided a written informed consent.
Disclosures
In this manuscript, there are no disclosures need to be reported by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, S., Zuo, H., Li, W. et al. The correlations between psychological distress, cognitive impairment and quality of life in patients with brain metastases after whole-brain radiotherapy. Clin Transl Oncol 25, 207–217 (2023). https://doi.org/10.1007/s12094-022-02927-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02927-3