Abstract
Purpose
FNDC4 gene encodes the fibronectin type III domain-containing 4 protein. Elevated expression of FNDC4 has been associated with poor prognosis in several types of cancer. There are no studies that have evaluated the prognostic capacity of FNDC4 in patients with head and neck cancer (HNSCC). The aim of our study was to analyze the relationship between the transcriptional expression of FNDC4 and prognosis in HNSCC patients.
Methods
We determined the transcriptional expression of FNDC4 in 67 patients with advanced-stage HNSCC (III–IV) treated with chemoradiotherapy. The FNDC4 expression was categorized according to the disease-specific survival with a recursive partitioning analysis.
Results
There were significant differences in disease-specific survival as a function of the level of FNDC4 transcriptional expression. The 5-year disease-specific survival for patients with high FNDC4 expression (n = 44, 65.7%) was 32.9% (95% CI: 16.5–49.3%), and for patients with low expression (n = 23, 34.3%) it was 85.4% (95% CI: 70.2–100%) (P = 0.0001). Patients with a high FNDC4 expression had poorer local (P = 0.097), regional (P = 0.008), and distant (0.034) recurrence-free survival. The results of a multivariate analysis showed that patients with a high FNDC4 expression had a 6.15-fold increased risk of death as a consequence of the HNSCC (95% CI: 1.71–22.06).
Conclusion
FNCF4 transcriptional expression was significantly related to the disease-specific survival of HNSCC patients treated with chemoradiotherapy. Patients with elevated FNDC4 expression had a significant decrease in disease-specific survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the clinical guidelines, one of the treatments of choice in patients with advanced-stage head and neck squamous cell carcinoma (HNSCC) is chemoradiotherapy [1]. After treatment with chemoradiotherapy, a variable proportion of patients will suffer a local, regional or distant recurrence. The availability of biomarkers that indicate which patients have a higher risk of recurrence would allow us to consider alternative therapies or to evaluate the convenience of an intensification of the treatment.
In the results of a study carried out in our center with cDNA arrays comparing the transcriptome of patients with HNSCC treated with radiotherapy or chemoradiotherapy as a function of the response to treatment (unpublished data), one of the genes that appeared to be differentially expressed was the FNDC4.
The FNDC4 gene encodes the fibronectin type III domain-containing 4 protein (also called Frcp1). FNDC4 is a member of the fibronectin type III domain family. This family consists of five proteins (FNDC1-5), of which the best known is FNDC5/irisin, which is a membrane protein expressed in the muscle, released during exercise, and which regulates the transformation of adipose tissue into brown fat, modulating energy expenditure [2].
FNDC4 was characterized by Bosma et al. [3], described as a transmembrane protein with the capacity of decreasing the expression of pro-inflammatory cytokines in a murine model of inflammatory bowel disease. FNDC4 has an extracellular domain that can be cleaved releasing a functionally active peptide [4].
In a study in patients with hepatocarcinoma, Wang et al. described that immunohistochemical overexpression of FNDC4 was associated with decreased survival, promoting in vitro an increase in migration and invasion [5].
To our knowledge, there are no studies that have evaluated the prognostic capacity of FNDC4 in patients with HNSCC. The present study aims to analyze the prognostic capacity of FNDC4 transcriptional expression in patients with HNSCC treated with chemoradiotherapy.
Material and methods
Patients
The present study was performed retrospectively based on the analysis of biopsy specimens obtained from the primary tumor site before any type of treatment in a cohort of 67 patients with advanced-stage HNSCC (III–IV) treated with chemoradiotherapy during the period 2008–2016. Clinical information was obtained from a database that prospectively collects data on all patients with malignant tumors diagnosed in our center since 1985 [6]. All patients included in the study were assessed by the Oncology Board of our center, and treatment with chemoradiotherapy were proposed according to the institutional therapeutic protocols. Table 1 shows the characteristics of the patients included in the study.
The human papillomavirus (HPV) status infection was assessed for patients with oropharyngeal carcinomas diagnosed up to 2012 by detection of viral DNA with SPF-10 RT-PCR, using the LiPA25-vl reverse hybridization assay for genotyping. From 2013 onwards, the CLART HPV-2 PCR assay was used. For all positive HPV-DNA samples, we evaluated the immunohistochemical expression of p16INK4a. Nuclear and cytoplasmic staining intensity was determined, and specimens with intense and diffuse staining of more than 70% of the tumor tissue were considered p16INK4a positive. We considered HPV-related tumors (HPV-positive) only when both the presence of viral DNA together with immunopositivity to p16INK4a was present. Given the interaction between tobacco and alcohol consumption, a combined variable of toxic consumption was created with three categories: no consumption; moderate consumption (< 20 cigarettes/day and/or < 80 g alcohol/day); and severe consumption (≥ 20 cigarettes/day or ≥ 80 g alcohol/day).
Radiotherapy was administered at a dose of 70–72 Gy on the primary location of the tumor, 50 Gy on the lymph node areas at risk in N0 patients, and 70–72 Gy on the affected lymph node areas in N + patients. Radiotherapy was planned using 3D conformal fields until 2010, and IMRT (Intensity Modulated Radiation Therapy) from 2011 onwards, with linear accelerators as the radiation source. Chemotherapy consisted of two to three cycles of cisplatin at a dose of 100 mg/m2 every 21 days and initiated simultaneously with radiotherapy. Neck dissection was included in the therapeutic scheme in 16 patients, after completing treatment with chemoradiotherapy.
The study was approved by the Institutional Review Board of the center and was conducted following the principles outlined in the Declaration of Helsinki.
Transcriptional analysis
The biopsy samples obtained from each patient were immediately enclosed in RNA-later (Quiagen GmbH, Hilden, Germany) to prevent mRNA degradation, and stored at − 80 °C until processing. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions. The cDNA was obtained by reverse transcription of 1 µg of RNA with High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, USA), and transcriptional expression of FNDC4 and beta-actin as an endogenous control was assessed by RT-PCR on an ABI Prism 7000 using validated assays (TaqMan Gene Expression Assays; Applied Biosystems).
The Cancer Genome Atlas (TCGA) data
To compare the transcriptional expression of FNDC4 between the tumor and a sample of healthy mucosa, we analyzed the data included in The Cancer Genome Atlas, which is an open-access database [7] that includes the transcriptome of a total of 520 head and neck carcinomas from different locations, as well as a total of 44 samples of normal mucosa. For the present study, the transcriptional expression of FNDC4 from normal mucosal samples and tumor samples corresponding to the same patient was analyzed. Additionally, we analyzed the transcriptional expression of 42 oropharyngeal tumor samples included in TCGA with known HPV status.
Statistical analysis
The distribution of FNDC4 transcriptional expression values did not meet normality criteria (Kolmogorov–Smirnov test P = 0.017), so measures of central tendency were expressed using the median value, and nonparametric techniques were used in the comparison of expression levels. The transcriptional expression rates of FNDC4 were compared according to gender, toxic consumption, location of the primary tumor, local and regional extension category, histologic grade, HPV status, and disease-specific survival.
The continuous value of FNDC4 expression was categorized with a recursive partition analysis using a classification and regression tree model, considering the disease-specific survival as the dependent variable. We calculated the disease-specific survival according to the categories obtained with the recursive partitioning analysis with the Kaplan–Meier method. The differences between survival curves were analyzed with the log-rank test. A multivariate analysis was carried out with the Cox proportional hazards model, considering the disease-specific survival as the dependent variable, and the location of the primary tumor, the local and regional extension category, and the FNDC4 expression category as independent variables.
Results
No significant differences appeared in the transcriptional expression value of FNDC4 according to gender (P = 0.629), toxic consumption (P = 0.237), primary tumor location (P = 0.175), local extension (P = 0.348), regional extension (P = 0.703), or histological grade (0.938). Table 1 in the Supplementary Material shows the median value of FNDC4 transcriptional expression according to the different variables analyzed.
HPV status information was available for 38 of the 46 patients with oropharyngeal carcinomas. Ten patients (26.3%) had an HPV-positive tumor. Patients with HPV-positive oropharyngeal carcinomas had significantly lower FNDC4 transcriptional expression values than those with HPV-negative tumors (P = 0.044). Figure 1 in the Supplementary Material shows the distribution in the FNDC4 expression values as a function of the HPV status. Information regarding HPV status was available for 42 patients with oropharyngeal carcinomas included in TCGA, of which 34 (81.0%) were considered HPV-positive. Again, HPV-positive tumors had significantly lower FNDC4 transcriptional expression values (P = 0.006). Figure 2 in the Supplementary Material shows the distribution of FNDC4 expression values of oropharyngeal carcinomas included in TCGA as a function of the HPV status.
The transcriptional expression of FNDC4 in tumor and normal mucosa were compared in the samples included in TCGA. Transcriptional expression in normal mucosa samples was significantly higher than in tumor tissue (P = 0.0001). Figure 3 in the Supplementary Material shows the distribution of FNDC4 transcriptional expression values in tumor and normal mucosa samples included in TCGA.
During the follow-up period, 26 patients (38.8%) had local recurrence of the tumor, 11 patients (16.4%) had a regional recurrence, and 12 patients (17.9%) had distant metastases. Twenty-nine patients (43.3%) died as a result of tumor progression. Patients who died as a consequence of the tumor had significantly higher transcriptional expression levels of FNDC4 (P = 0.007). Figure 1 shows the distribution of FNDC4 expression as a function of the disease-specific survival.
Considering the disease-specific survival as the dependent variable, the recursive partitioning analysis classified patients into two categories according to the FNDC4 expression. The 5-year disease-specific survival for patients with high FNDC4 expression (n = 44, 65.7%) was 32.9% (95% CI: 16.5–49.3%), and for patients with low expression (n = 23, 34.3%) it was 85.4% (95% CI: 70.2–100%). There were significant differences in disease-specific survival as a function of the FNDC4 transcriptional expression category (P = 0.0001). Figure 2 shows the disease-specific survival curves according to the FNDC4 transcriptional expression category.
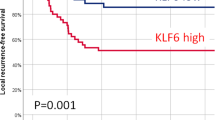
Patients with a high FNDC4 expression had poorer local (P = 0.097), regional (P = 0.008), and distant (0.034) recurrence-free survival. Figure 4 in the supplementary material shows the local, regional, and distant recurrence-free survival curves as a function of the FNDC4 expression category.
Table 2 shows the result of a multivariate analysis considering the disease-specific survival as the dependent variable. According to the results of this multivariate analysis, the variables that were significantly related to a decrease in the disease-specific survival were the location of the tumor in the hypopharynx and a high FNDC4 transcriptional expression rate. Relative to patients with low FNDC4 expression, patients with a high expression had a 6.15-fold increased risk of death as a consequence of the HNSCC (95% CI: 1.71–22.06).
Discussion
According to our results, transcriptional expression of FNDC4 was significantly related to disease-specific survival in HNSCC patients treated with chemoradiotherapy. Patients with elevated FNDC4 expression had an increased risk of recurrence. This increased risk reached statistical significance in the case of regional and distant disease control.
FNDC4 is a membrane protein described by Bosma et al. [3]. Like FNDC5/irisin, FNDC4 has an extracellular portion at the N-terminal end that can be cleaved, secreting a peptide with an immunomodulatory activity. This peptide has the capacity of reducing the inflammatory process in a murine model of inflammatory bowel disease. In a further study, Lee et al. described the ability of FNDC4 to ameliorate hyperlipidemia-induced insulin resistance [8].
In a recent study, Frühbeck et al. [9] found that patients with obesity had reduced plasma levels of FNDC5 and FNDC4, and that administration of FNDC5 or FNDC4 induced a decrease in the expression in the adipose tissue of genes encoding receptors for SARS-CoV-2 viruses such as ACE2, CD147 or NRP1. This could justify one of the mechanisms by which obese patients are more susceptible to COVID-19 infection.
There is very limited information linking FNDC4 activity to tumor development. In a study of a cohort of 205 patients with hepatocarcinoma in which FNDC4 expression was assessed by immunohistochemistry, Wang et al. [5] found that tumors with an elevated expression level were less differentiated, and had more microvascular invasion, a higher risk of tumor recurrence (P = 0.277) and poorer survival (P = 0.002). In an in vitro study carried out with hepatic carcinoma cell lines they found that induction of FNDC4 expression significantly increased the capacity for migration and invasion, and how this activity was a consequence of activation of the PI3K/Akt pathway. In another study performed with bovine muscle satellite stem cells, Wang et al. [10] described an increase in the migratory capacity of this cell type induced by FNDC4.
To our knowledge, there are no studies that have related FNDC4 expression with prognosis in patients with HNSCC. When analyzing the data included in TCGA we observed that HNSCC samples had a significantly lower transcriptional expression of FNDC4 than those corresponding to normal mucosa. In an analysis of data included in another database that provides molecular tumor information (GEO Data Sets), Wuensch et al. [11] also described a reduction in the transcriptional expression of FNDC4 in colorectal carcinoma relative to healthy tissue samples.
The data included in TCGA showed that HNSCC patients with elevated levels of FNDC4 had lower overall survival, although the differences did not reach statistical significance (P = 0.13). It should be considered that the characteristics of the patients included in TCGA are very different from those of the patients analyzed in our study, with a large proportion of patients with oral cavity carcinomas and a large majority of patients treated with surgery. In other tumor models included in TCGA, such as endometrial or urothelial carcinoma, an increase in the transcriptional expression of FNDC4 was associated with a significant reduction in survival.
One element to highlight is the differential expression of FNDC4 in patients with oropharyngeal carcinomas according to HPV status. Both in our patients and the sample of patients included in TCGA, HPV-positive tumors had a significantly lower transcriptional expression value of FNDC4. We interpret this finding as a consequence of the different mechanisms of carcinogenesis of oropharyngeal tumors according to HPV infection.
This study has several limitations associated with its retrospective nature, including a limited and heterogeneous sample of tumors with different locations and extensions. All patients were treated exclusively with chemoradiotherapy, which restricts the possibility of transferring the results obtained to patients receiving other types of treatment. In addition, FNDC4 expression was analyzed only at the transcriptional level, lacking information on possible post-transcriptional regulation processes.
However, our study points out for the first time the relationship between FNDC4 expression and treatment response to chemoradiotherapy in HNSCC patients. Further validation studies are necessary before considering FNDC4 transcriptional expression as a biomarker with prognostic ability in HNSCC patients.
Conclusion
TNCF4 transcriptional expression was significantly related to the disease-specific survival of HNSCC patients treated with chemoradiotherapy. Patients with elevated FNDC4 expression had a significant decrease in disease-specific survival.
References
Mesia R, Iglesias L, Lambea J, Martínez-Trufero J, Soria A, Taberna M, Trigo J, Chaves M, García-Castaño A, Cruz J. SEOM clinical guidelines for the treatment of head and neck cancer (2020). Clin Transl Oncol. 2020;23:913–21.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Bosma M, Gerling M, Pasto J, Georgiadi A, Graham E, Shilkova O, Iwata Y, Almer S, Söderman J, Toftgård R, Wermeling F, Boström EA, Boström PA. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nat Commun. 2016;7:11314.
Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83.
Wang B, Zheng B, Lu Y, Huang D, Liu J, Song J, Zheng S. FNDC4 acts as an extracellular factor to promote the invasiveness of hepatocellular carcinoma partly via the PI3K/Akt signaling pathway. Cancer Med. 2021;10:7242–52.
León X, Orús C, Quer M. Diseño, mantenimiento y explotación de una base de datos oncológica para pacientes con tumores malignos de cabeza y cuello. Acta Otorrinolaringol Esp. 2002;53:185–90.
The Cancer Genome Atlas. https://tcga-data.nci.nih.gov/tcga.
Lee W, Yun S, Choi GH, Jung TW. Fibronectin Type III Domain Containing 4 attenuates hyperlipidemia-induced insulin resistance via suppression of inflammation and ER stress through HO-1 expression in adipocytes. Biochem Biophys Res Commun. 2018;502:129–36.
Frühbeck G, Catalán V, Valentí V, Moncada R, Gómez-Ambrosi J, Becerril S, Silva C, Portincasa P, Escalada J, Rodríguez A. FNDC4 and FNDC5 reduce SARS-CoV-2 entry points and spike glycoprotein S1-induced pyroptosis, apoptosis, and necroptosis in human adipocytes. Cell Mol Immunol. 2021;18:2457–9.
Wang Z, Wang Z, Pang Y, Tong H, Yan Y, Li S, Li S. Fibronectin type III domain-containing 4 promotes the migration and differentiation of bovine skeletal muscle-derived satellite cells via focal adhesion kinase. Cell Adh Migr. 2020;14:153–64.
Wuensch T, Wizenty J, Quint J, Spitz W, Bosma M, Becker O, Adler A, Veltzke-Schlieker W, Stockmann M, Weiss S, Biebl M, Pratschke J, Aigner F. Expression Analysis of fibronectin type III domain-containing (FNDC) genes in inflammatory bowel disease and colorectal cancer. Gastroenterol Res Pract. 2019;2019:3784172.
Funding
This study was supported by a grant from Instituto de Salud Carlos III (FIS PI19/01661 to XL). Fondo Europeo de Desarrollo Regional (FEDER), A Way to Build Europe.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the paper in conception, design, and writing. The publication has been approved by all (co-)authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical approval
The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the Hospital de Sant Pau Ethics Committee for Scientific Research (IIBSP-CCC-14–93).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Camacho, M., León, X., Pujol, A. et al. Prognostic value of transcriptional expression of fibronectin type III domain-containing 4 (FNDC4) in head and neck carcinoma patients treated with chemoradiotherapy. Clin Transl Oncol 24, 2175–2180 (2022). https://doi.org/10.1007/s12094-022-02870-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02870-3