Abstract
Purpose
Globally, lung cancer remains the most commonly diagnosed cancer and the leading cause of cancer-related mortality. Lung adenocarcinoma (LUAD) is a common subtype of lung cancer and carries a poor prognosis. Treatment outcomes biomarkers in LUAD are critical, and there is currently a paucity of data; therefore, there is a need for novel biomarkers and newer therapeutic targets.
Methods
Bayesian analysis was used to obtain the whole-genome t value of LUAD. Gene set enrichment analysis (GSEA) was conducted to obtain the normalized enrichment scores (NES) of the whole genome, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was analyzed using the Gene Set Analysis Toolkit. Herein, we investigated the PPP1R14D expression level at the protein level in LUAD and the impact of PPP1R14D knockdown on the proliferation and apoptosis of LUAD cells in vitro.
Results
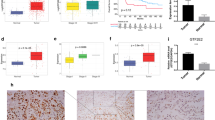
A total of 483 LUAD samples and 59 normal control samples were included, and 904 differentially expressed genes (DEGs) and 504 LUAD-related genes reported in the literature were obtained. The DEGs showed that PPP1R14D was the most significantly up-regulated gene. Western blot of 30 cases of LUAD tissue and adjacent normal tissue also found that PPP1R14D was significantly highly expressed in cancer tissues. Lentivirus-mediated shRNA strategy effectively inhibited PPP1R14D expression in human LUAD cells DMS53, while PPP1R14D knockdown induced apoptosis and cell proliferation in DMS53 cells.
Conclusion
Abnormally up-regulated PPP1R14D promotes the survival and proliferation of tumor cells in human LUAD and may serve as a therapeutic and diagnostic target for LUAD.





Similar content being viewed by others
References
Qu Y, Cheng B, Shao N, et al. Prognostic value of immune-related genes in the tumor microenvironment of lung adenocarcinoma and lung squamous cell carcinoma. Aging (Albany NY). 2020;12:4757–77.
Dong S, Men W, Yang S, et al. Identification of lung adenocarcinoma biomarkers based on bioinformatic analysis and human samples. Oncol Rep. 2020;43:1437–50.
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89.
Shi J, Hua X, Zhu B, et al. Somatic Genomics and Clinical Features of Lung Adenocarcinoma: A Retrospective Study. PLoS Med. 2016;13: e1002162.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16:341–55.
Li Y, Ge D, Gu J, et al. A large cohort study identifying a novel prognosis prediction model for lung adenocarcinoma through machine learning strategies. BMC Cancer. 2019;19:886.
Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103-115.
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Muller IB, de Langen AJ, Giovannetti E, et al. Anaplastic lymphoma kinase inhibition in metastatic non-small cell lung cancer: clinical impact of alectinib. Onco Targets Ther. 2017;10:4535–41.
Marchio C, Balmativola D, Castiglione R, et al. Predictive Diagnostic Pathology in the Target Therapy Era in Breast Cancer. Curr Drug Targets. 2017;18:4–12.
Zhang L, Zhang Z, Yu Z. Identification of a novel glycolysis-related gene signature for predicting metastasis and survival in patients with lung adenocarcinoma. J Transl Med. 2019;17:423.
Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–42.
Hagel C, Dornblut C, Schulz A, et al. The putative oncogene CPI-17 is up-regulated in schwannoma. Neuropathol Appl Neurobiol. 2016;42:664–8.
Cheng Z, Wen Y, Liang B, et al. Gene expression profile-based drug screen identifies SAHA as a novel treatment for NAFLD. Mol Omics. 2019;15:50–8.
Hou L, Song Z, Xu Z, et al. Folate-Mediated Targeted Delivery of siPLK1 by Leucine-Bearing Polyethylenimine. Int J Nanomedicine. 2020;15:1397–408.
Qu G, Ma Z, Tong W, et al. LncRNA WWOX-AS1 inhibits the proliferation, migration and invasion of osteosarcoma cells. Mol Med Rep. 2018;18:779–88.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–7.
Ito M, Nakano T, Erdodi F, et al. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209.
Grassie ME, Moffat LD, Walsh MP, et al. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch Biochem Biophys. 2011;510:147–59.
Woodsome TP, Eto M, Everett A, et al. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–64.
Eto M, Bock R, Brautigan DL, et al. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–58.
Karthik D, Ravikumar S. Characterization of the brain proteome of rats with diabetes mellitus through two-dimensional electrophoresis and mass spectrometry. Brain Res. 2011;1371:171–9.
Zhang W, Shang S, Yang Y, et al. Identification of DNA methylation-driven genes by integrative analysis of DNA methylation and transcriptome data in pancreatic adenocarcinoma. Exp Ther Med. 2020;19:2963–72.
Lokk K, Vooder T, Kolde R, et al. Methylation markers of early-stage non-small cell lung cancer. PLoS ONE. 2012;7: e39813.
Funding
This study is funded by Qiqihar City Science and Technology Plan Project (SFZD-2019152).
Author information
Authors and Affiliations
Contributions
YT and ZG conceived and designed the study, and drafted the manuscript. YT, LG, YQ and YW collected, analyzed and interpreted the data. YT and LG revised the manuscript for important intellectual content. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of the Second Affiliated Hospital of Qiqihar Medical College and conducted in accordance with the ethical standards.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, Y., Guan, L., Qian, Y. et al. Effect of PPP1R14D gene high expression in lung adenocarcinoma knocked out on proliferation and apoptosis of DMS53 cell. Clin Transl Oncol 24, 1914–1923 (2022). https://doi.org/10.1007/s12094-022-02842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02842-7