Abstract
Objective
Emerging studies highlight the crucial effects of microRNAs on cancer initiation and malignant progression of various tumors. This study focused on the biological effect of miR-377-3p on CUL1 and epithelial–mesenchymal transition (EMT) and Wnt/β-catenin pathways in osteosarcoma (OS).
Methods
We performed quantitative real-time polymerase chain reaction (qRT-PCR) to analyze miR-377-3p and CUL1 expression levels in OS tissues and MG-63 cells. Then, cell counting kit (CCK)-8 and Transwell assay were used to examine the functions of miR-377-3p in OS cell growth and metastasis abilities. Meanwhile, luciferase reporter assay was used to validate CUL1 as direct target of miR-377-3p. qRT-PCR and Western blot were then carried out to detect the impact of miR-377-3p on EMT and Wnt/β-catenin pathways. Tumor xenograft models were established to further examine the effects of miR-377-3p on OS tumorigenesis in vivo.
Results
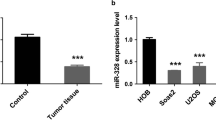
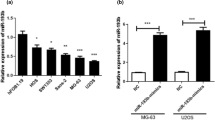
miR-377-3p downregulation was frequently identified in OS tissues and cells, which was associated with worse prognosis of OS patients. Functional experiments showed miR-377-3p restoration could dramatically repress OS cell growth and migration by regulation of EMT and Wnt/β-catenin pathways. Moreover, luciferase reporter assay revealed that CUL1 acted as a functional target of miR-377-3p. Additionally, the elevated CUL1 expressions in OS tissues also indicated poor prognosis of OS patients. Furthermore, the OS tumor growth was also obviously inhibited by miR-377-3p overexpression in vivo.
Conclusions
Collectively, all the above findings revealed that miR-377-3p exerted anti-OS functions via CUL1 and EMT and Wnt/β-catenin pathways. These results may contribute to the development of clinical OS treatment.





Similar content being viewed by others
References
Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92.
Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–30.
Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34.
Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci. 2020;21:6885.
Bajpai J, Chandrasekharan A, Simha V, Talreja V, Karpe A, Pandey N, Singh A, Rekhi B, Vora T, Ghosh J, Banavali S, Gupta S. Outcomes in treatment-naive patients with metastatic extremity osteosarcoma treated with OGS-12, a novel non-high-dose methotrexate-based, dose-dense combination chemotherapy, in a tertiary care cancer center. J Glob Oncol. 2018;4:1–10.
Zhang Y, Yang J, Zhao N, Wang C, Kamar S, Zhou Y, He Z, Yang J, Sun B, Shi X, Han L, Yang Z. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16:6228–37.
Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, Bonvalot S, Boukovinas I, Bovee J, Brennan B, Brodowicz T, Broto JM, Brugieres L, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv79–95.
Wang S, Gao H, Zuo J, Gao Z. Cyclooxygenase-2 expression correlates with development, progression, metastasis, and prognosis of osteosarcoma: a meta-analysis and trial sequential analysis. FEBS Open Bio. 2019;9:226–40.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Ji Q, Xu X, Song Q, Xu Y, Tai Y, Goodman SB, Bi W, Xu M, Jiao S, Maloney WJ, Wang Y. miR-223-3p inhibits human osteosarcoma metastasis and progression by directly targeting CDH6. Mol Ther. 2018;26:1299–312.
Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, Grieb P, Rutkowski P. Molecular biology of osteosarcoma. Cancers (Basel). 2020;12:2130.
Xia P, Gu R, Zhang W, Shao L, Li F, Wu C, Sun Y. MicroRNA-377 exerts a potent suppressive role in osteosarcoma through the involvement of the histone acetyltransferase 1-mediated Wnt axis. J Cell Physiol. 2019;234:22787–98.
Huang YF, Lu L, Shen HL, Lu XX. LncRNA SNHG4 promotes osteosarcoma proliferation and migration by sponging miR-377–3p. Mol Genet Genomic Med. 2020;8:e1349.
Bu N, Dong Z, Zhang L, Zhu W, Wei F, Zheng S. CircPVT1 regulates cell proliferation, apoptosis and glycolysis in hepatocellular carcinoma via miR-377/TRIM23 axis. Cancer Manag Res. 2020;12:12945–56.
Yu R, Cai L, Chi Y, Ding X, Wu X. miR377 targets CUL4A and regulates metastatic capability in ovarian cancer. Int J Mol Med. 2018;41:3147–56.
Huang YF, Zhang Z, Zhang M, Chen YS, Song J, Hou PF, Yong HM, Zheng JN, Bai J. CUL1 promotes breast cancer metastasis through regulating EZH2-induced the autocrine expression of the cytokines CXCL8 and IL11. Cell Death Dis. 2018;10:2.
Cheng Q, Yin G. Cullin-1 regulates MG63 cell proliferation and metastasis and is a novel prognostic marker of osteosarcoma. Int J Biol Markers. 2017;32:e202–9.
Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW Jr, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–16.
Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):89–92.
Song M, Wang Y, Zhang Z, Wang S. PSMC2 is up-regulated in osteosarcoma and regulates osteosarcoma cell proliferation, apoptosis and migration. Oncotarget. 2017;8:933–53.
Xie HF, Liu YZ, Du R, Wang B, Chen MT, Zhang YY, Deng ZL, Li J. miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1. Cell Death Dis. 2017;8:e2663.
Jang SM, Redon CE, Aladjem MI. Chromatin-bound cullin-ring ligases: regulatory roles in DNA replication and potential targeting for cancer therapy. Front Mol Biosci. 2018;5:19.
Sweeney MA, Iakova P, Maneix L, Shih FY, Cho HE, Sahin E, Catic A. The ubiquitin ligase Cullin-1 associates with chromatin and regulates transcription of specific c-MYC target genes. Sci Rep. 2020;10:13942.
Liu J, Su S, He H, Wang H, Zhang D. Effects of Cullin1 on the Biological Characteristics of Lung AdenocarcinomaA549 and H1395 Cells. Zhongguo Fei Ai Za Zhi. 2021;24:69–77.
Deng J, Chen W, Du Y, Wang W, Zhang G, Tang Y, Qian Z, Xu P, Cao Z, Zhou Y. Synergistic efficacy of Cullin1 and MMP-2 expressions in diagnosis and prognosis of colorectal cancer. Cancer Biomark. 2017;19:57–64.
Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng XJ, Cao MT, Zhong CY, Liu Y, Shan H, Jiang GM. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol Cancer. 2019;18:101.
Lavin DP, Tiwari VK. Unresolved complexity in the gene regulatory network underlying EMT. Front Oncol. 2020;10:554.
Lei Y, Chen L, Zhang G, Shan A, Ye C, Liang B, Sun J, Liao X, Zhu C, Chen Y, Wang J, Zhang E, Deng L. MicroRNAs target the Wnt/betacatenin signaling pathway to regulate epithelialmesenchymal transition in cancer (Review). Oncol Rep. 2020;44:1299–313.
Guo Q, Qin W. DKK3 blocked translocation of beta-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J Cell Mol Med. 2015;19:2832–41.
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, Wu J, Li M. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–79.
Peng L, Liu Z, Xiao J, Tu Y, Wan Z, Xiong H, Li Y, Xiao W. MicroRNA-148a suppresses epithelial-mesenchymal transition and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt/beta-catenin signaling pathway. Oncol Rep. 2017;38:301–8.
Jin Y, Wang J, Han J, Luo D, Sun Z. MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/beta-cadherin signaling pathway. Exp Cell Res. 2017;360:210–7.
Author information
Authors and Affiliations
Contributions
KL, LL and QL are responsible for the conception or design of the work. QO, LJ and GW contribute the acquisition, analysis, or interpretation of data for the work. All authors finally approved the manuscript version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of Nanxishan Hospital of Guangxi Zhuang Autonomous Region and conducted in accordance with the ethical standards.
Informed consent
Subjects signed the informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, K., Liao, L., Liu, Q. et al. microRNA-377-3p inhibits osteosarcoma progression by targeting CUL1 and regulating Wnt/β-catenin signaling pathway. Clin Transl Oncol 23, 2350–2357 (2021). https://doi.org/10.1007/s12094-021-02633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02633-6