Abstract
Purpose
Endocrine therapy is a mainstay for the treatment of hormone receptor-positive breast cancer (BC); however, only a fraction of patients experience a pronounced response to antagonists of estrogen signaling. There is a need to identify predictors for efficacy of this treatment.
Methods
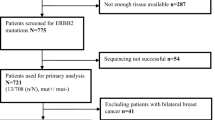
This study included 138 patients with newly diagnosed metastatic BC, who received upfront endocrine therapy. Archival biopsy specimens were tested for CCND1 and FGFR1 gene amplification and mRNA expression by PCR-based methods.
Results
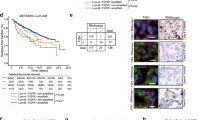
CCND1 and FGFR1 amplification was detected in 24 (17.9%) and 28 (20.9%) of 134 evaluable cases, respectively; 9 carcinomas had concurrent alterations of these two genes. Presence of amplification in at least one locus was more common in tumors of higher grade (p = 0.018) and was associated with higher Ki-67 proliferation index (p = 0.036). CCND1 gene amplification was associated with shorter progression-free survival (PFS) in patients receiving aromatase inhibitors (AI) [16.0 months vs. 32.4 months, HR = 3.16 (95% CI 1.26–7.93), p = 0.014]. FGFR1 status did not significantly affect PFS of AI-treated women; however, objective response to AI was observed less frequently in FGFR1-amplified BC as compared to cases with normal FGFR1 copy number [2/15 (13.3%) vs. 22/46 (47.8%), p = 0.031]. Meanwhile, CCND1/FGFR1 gene status did not influence the outcome of tamoxifen-treated patients.
Conclusion
Presence of CCND1 and/or FGFR1 amplification is associated with worse outcomes of AI therapy in patients with metastatic BC.



Similar content being viewed by others
Data availability
The data generated and analyzed during the current study are included in the article and its supplementary information files.
References
Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–98.
Jirawatnotai S, Hu Y, Michowski W, Elias JE, Becks L, Bienvenu F, et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature. 2011;474:230–4.
Casimiro MC, Di Sante G, Crosariol M, Loro E, Dampier W, Ertel A, et al. Kinase-independent role of cyclin D1 in chromosomal instability and mammary tumorigenesis. Oncotarget. 2015;6:8525–38.
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–7.
Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res. 2012;14:R57.
Roy PG, Pratt N, Purdie CA, Baker L, Ashfield A, Quinlan P, et al. High CCND1 amplification identifies a group of poor prognosis women with estrogen receptor positive breast cancer. Int J Cancer. 2010;127:355–60.
Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5:2069–76.
Bièche I, Olivi M, Noguès C, Vidaud M, Lidereau R. Prognostic value of CCND1 gene status in sporadic breast tumours, as determined by real-time quantitative PCR assays. Br J Cancer. 2002;86:580–6.
Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, Lax S, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14:1767–74.
Beca F, Pereira M, Cameselle-Teijeiro JF, Martins D, Schmitt F. Altered PPP2R2A and cyclin D1 expression defines a subgroup of aggressive luminal-like breast cancer. BMC Cancer. 2015;15:285.
Ahlin C, Lundgren C, Embretsén-Varro E, Jirström K, Blomqvist C, Fjällskog M-L. High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers. Breast Cancer Res Treat. 2017;164:667–78.
Stendahl M, Kronblad A, Rydén L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004;90:1942–8.
Jirström K, Stendahl M, Rydén L, Kronblad A, Bendahl PO, Stål O, et al. Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res. 2005;65:8009–166.
Ahnström M, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O. Role of cyclin D1 in ErbB2-positive breast cancer and tamoxifen resistance. Breast Cancer Res Treat. 2005;91:145–51.
Brown LA, Johnson K, Leung S, Bismar TA, Benítez J, Foulkes WD, et al. Co-amplification of CCND1 and EMSY is associated with an adverse outcome in ER-positive tamoxifen-treated breast cancers. Breast Cancer Res Treat. 2010;121:347–54.
Theillet C, Adelaide J, Louason G, Bonnet-Dorion F, Jacquemier J, Adnane J, et al. FGFRI and PLAT genes and DNA amplification at 8p12 in breast and ovarian cancers. Genes Chromosomes Cancer. 1993;7(4):219–26.
Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–94.
Tomiguchi M, Yamamoto Y, Yamamoto-Ibusuki M, Goto-Yamaguchi L, Fujiki Y, Fujiwara S, et al. Fibroblast growth factor receptor-1 protein expression is associated with prognosis in estrogen receptor-positive/human epidermal growth factor receptor-2-negative primary breast cancer. Cancer Sci. 2016;107:491–8.
Elbauomy Elsheikh S, Green AR, Lambros MB, Turner NC, Grainge MJ, Powe D, et al. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res. 2007;9:R23.
Formisano L, Stauffer KM, Young CD, Bhola NE, Guerrero-Zotano AL, Jansen VM, et al. Association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER+ breast cancer. Clin Cancer Res. 2017;23:6138–50.
Karlsson E, Waltersson MA, Bostner J, Pérez-Tenorio G, Olsson B, Hallbeck AL, et al. High-resolution genomic analysis of the 11q13 amplicon in breast cancers identifies synergy with 8p12 amplification, involving the mTOR targets S6K2 and 4EBP1. Genes Chromosomes Cancer. 2011;50:775–87.
Jang M, Kim E, Choi Y, Lee H, Kim Y, Kim J, et al. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012;14:R115.
Shi YJ, Tsang JY, Ni YB, Chan SK, Chan KF, Tse GM. FGFR1 is an adverse outcome indicator for luminal a breast cancers. Oncotarget. 2016;7:5063–73.
Yanus GA, Belyaeva AV, Ivantsov AO, Kuligina ESh, Suspitsin EN, Mitiushkina NV, et al. Pattern of clinically relevant mutations in consecutive series of Russian colorectal cancer patients. Med Oncol. 2013;30:686.
Mitiushkina NV, Iyevleva AG, Poltoratskiy AN, Ivantsov AO, Togo AV, Polyakov IS, et al. Detection of EGFR mutations and EML4-ALK rearrangements in lung adenocarcinomas using archived cytological slides. Cancer Cytopathol. 2013;121:370–6.
Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, et al. Cyclin D1 expression and patient outcome after tamoxifen therapy in estrogen receptor positive metastatic breast cancer. Oncol Rep. 2003;10:141–4.
Bostner J, Ahnström Waltersson M, Fornander T, Skoog L, Nordenskjöld B, Stål O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26:6997–7005.
Keilty D, Buchanan M, Ntapolias K, Aleynikova O, Tu D, Li X, et al. RSF1 and not cyclin D1 gene amplification may predict lack of benefit from adjuvant tamoxifen in high-risk pre-menopausal women in the MA.12 randomized clinical trial. PLoS ONE. 2013;8:e81740.
Giltnane JM, Hutchinson KE, Stricker TP, Formisano L, Young CD, Estrada MV, et al. Genomic profiling of ER+ breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med. 2017;9(402):eaai7993.
Drago JZ, Formisano L, Juric D, Niemierko A, Servetto A, Wander SA, et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR(+)) breast cancer. Clin Cancer Res. 2019;25(21):6443–511.
Funding
The study was funded by the Russian Science Foundation (grant number 19-15-00207).
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: ENI, AGI; Methodology: ADM, SEK; Formal analysis and investigation: SNA, MMK, VVP, AOI, VMM; Writing—original draft preparation: AGI; Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional Ethical Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aleksakhina, S.N., Kramchaninov, M.M., Mikushina, A.D. et al. CCND1 and FGFR1 gene amplifications are associated with reduced benefit from aromatase inhibitors in metastatic breast cancer. Clin Transl Oncol 23, 874–881 (2021). https://doi.org/10.1007/s12094-020-02481-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02481-w