Abstract
Purpose
The purpose of this study was to explore the differences between stage T2N0M0 and stage T1N1M0 gastric cancer (GC) and to identify the necessity of adjuvant treatment (AT) for these stages.
Methods
Between years 2004 and 2015, 1971 stage IB GC patients who underwent radical surgery were recruited using the Surveillance, Epidemiology and End Results database. We conducted univariate/multivariate analyses, the propensity score matching and evaluated gastric cancer-specific survival (GCSS) and overall survival (OS) with the log-rank test.
Results
T1N1M0 had a significantly worse survival than T2N0M0 in both GCSS and OS before and after the propensity score matching. Examined lymph nodes (ELN) ≤ 15 and T1N1M0 were independent risk factors for worse GCSS and OS in stage IB GC. The absence of adjuvant chemotherapy (CT) was an independent risk factor for worse GCSS and OS in T1N1M0 but not in T2N0M0. AT demonstrated similar GCSS and OS with surgery alone (SA) for T2N0M0 but better survival for T1N1M0. Compared to CT and adjuvant chemoradiotherapy (CRT) group, SA demonstrated significantly worse GCSS and OS for T1N1M0. There was no significant difference between CT and CRT in both T2N0M0 and T1N1M0 stages. T2N0M0 had a better survival than T1N1M0 in ELN ≤ 15 subgroup. However, similar survival was demonstrated in ELN > 15 subgroup.
Conclusions
T2N0M0 GC has a better survival rate than T1N1M0 GC when ELN are ≤ 15. Moreover, T2N0M0 GC may not benefit from AT. T1N1M0 GC requires CT but not adjuvant radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fourth leading cause of cancer-related death worldwide [1]. Gastric cancer patients, survival can be predicted based on the 8th edition of the American Joint Committee on Cancer (AJCC) tumor–lymph node–metastasis (TNM) classification, which provides important prognostic instruction for further treatment [2]. According to the National Comprehensive Cancer Network (NCCN), adjuvant chemotherapy (CT) is recommended for stage T1N1M0 (invading the mucosa or submucosa, having 1 or 2 positive lymph nodes and no distant metastasis) GC after the curative resection [3]. On the other hand, while CT is not recommended for all stage T2N0M0 (invading the muscularis propria and having no positive lymph node and no distant metastasis) patients, it is recommended for high-risk patients with poorly differentiated or higher-grade cancer, lymphatic and/or blood vessel invasion, perineural invasion, or who are under 50 years of age. According to the Japanese Gastric Cancer Treatment Guidelines, observation alone is recommended without any adjuvant treatment (AT) after curative resection for both T1N1M0 and T2N0M0 GC [4]. Currently, two main randomized controlled trial (RCT) studies exist for AT, ACTS-GC trial [5] (included stage II-III GC) and CLASSIC trial [6] (including stage II-IIIB GC) in Asia. However, these studies do not address whether stage IB GC patients would benefit from AT following surgery. Thus, unlike stage II–III GC, there is no consensus on using AT for stage IB GC.
Kwon et al. reported a small gap in the 5-year survival between stage IB and IA GC [7]. Previous studies have found an overlapping survival curve between stage IB and stage IIA GC using the 7th/8th edition AJCC staging system [8,9,10]. Furthermore, Gold et at reported significantly different disease-specific survival at 5 years between stage T1N1M0 GC and stage T2N0M0 GC which are both stage IB GC [11]. In contrast, other reports demonstrated no differences in survival between the two subgroups [12,13,14,15]. Few reports exist on stage migration and treatment strategies of stage IB GC and the prognostic factors of stage IB remain unclear. If the similar prognosis of stages T2N0M0 and T1N1M0 were confirmed, some patients may benefit from sparing of treatment-induced toxicity. If a worse prognosis of the T2N0M0 stage is confirmed, AT would be recommended.
The primary aim of this study was to explore the differences between the stages T2N0M0 and T1N1M0 GC and to identify the necessity of AT in each stage using the Surveillance, Epidemiology and End Results (SEER) database.
Materials and methods
Patients selection
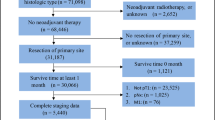
The data were derived from the SEER database of the US National Cancer Institute [SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 sub (1973–2015 varying)]. We identified eligible patients according to the inclusion criteria as follows: (1) All patients were diagnosed with stomach cancer from 2004 to 2015 by histopathological examination; (2) Pathologic confirmation was adenocarcinoma, mucin-producing adenocarcinoma, mucinous cyst-denocarcinoma, signet ring cell carcinoma, papillary adenocarcinoma, tubular adenocarcinoma, adenocarcinoma intestinal type, carcinoma diffuse type, adenocarcinoma with mixed type; (3) No distant metastasis; (4) The 6th edition AJCC stage was T1N1MO and T2aN0M0. Because all the patients included for this paper in SEER database were surgery performed, thus the TNM stage indicated pathological stage, i.e., pTNM stage.
The exclusion criteria were as follows: (1) patients survived < 1 month after surgery or survival was unknown; (2) Not receiving surgery or unknown if surgery performed; (3) Regional nodes examined were none or unknown; (4) Regional nodes positive were unknown; (5) Regional nodes positive were 3,4,5,6 when we used the searching term as T1N1 (1–6 positive nodes) M0 because all the staging data were updated and coded to conform to the 8th edition of the AJCC TNM staging system; (6) Tumor destruction; pathologic specimen unknown or no pathologic specimen. (The flow chart of searching process is shown in Fig. 1).
Statistical analysis and outcomes
The basic patient data from SEER database included year of diagnosis, age at diagnosis, gender, race, examined lymph nodes (ELN), family income, marital status, tumor size, tumor site, histological grade, histological type, Lauren’s type, surgery, adjuvant radiotherapy (RT), CT, T stage, N stage, M stage, TNM stage, survival months and cause of death.
The clinicopathological characteristics between T2N0M0 group and T1N1M0 group were compared by Chi squared test. Survival curves were performed using the Kaplan–Meier method and gastric cancer-specific survival (GCSS) and overall survival (OS) differences were determined by the log-rank test. Adjusted hazard ratios (HR) with 95% confidence interval (CI) were calculated using the Cox proportional regression model to estimate the prognostic factors. For all the statistical analyses, Stata 15.1 software (StataCorp, College Station, TX, USA) and SPSS statistical software, version 23 (SPSS, Chicago, IL, USA) were used. Since patients were assigned to different groups based on retrospective review rather than randomization, propensity score analysis was used to balance out different parameters. We matched one stage T2N0M0 patient to one stage T1N1M0 patient utilizing the EmpowerStats software (EmpowerStats, Boston, X&Y Solutions, USA) to calculate the propensity score and detect the matching efficiency for the balance. A two-sided P < 0.05 was considered as statistically significant.
Results
Demographic and tumor characteristics
This study identified 1971 (T2N0M0 = 1407, T1N1M0 = 564) patients from the SEER database who met the inclusion criteria (Table 1). The mean number of ELN was 15.33 and 1198 (60.78%) patients had < 15 ELN in the whole cohort. Significant differences were found between the two groups in the characteristics studied such as tumor size, histologic type, CT, and RT. The proportion of larger tumor (tumor size > 5 cm) was significantly higher in the T2N0M0 group (19.29% in T2N0M0 vs. 11.11% in T1N1M0, P < 0.001). In contrast, patients with T1N1M0 were more likely to have a higher proportion of signet ring cell carcinoma (17.38% in T1N1M0 vs. 13.86% in T2N0M0, P = 0.047), CT (53.37% in T1N1M0 vs. 29.71% in T2N0M0, P < 0.001), RT (39.72% in T1N1M0 vs. 20.11% in T2N0M0, P < 0.001) and smaller tumor (tumor size ≤ 2 cm) (37.23% in T1N1M0 vs. 28.32% in T2N0M0, P < 0.001). The other carcinoma characteristics were similarly distributed between the two groups.
Comparison of survival between T2N0M0 and T1N1M0
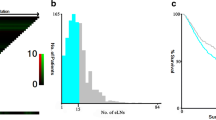
We evaluated the GCSS (in 1317 patients totally, 924 patients for T2N0M0 and 393 patients for T1N1M0) and OS (in 1971 patients, 1407 patients for T2N0M0 and 564 patients for T1N1M0) in T2N0M0 and in T1N1M0 group using the Kaplan–Meier analysis and log-rank test. We found that compared to T1N1M0, T2N0M0 had a better survival both in GCSS (P = 0.002) and OS (P = 0.042) (Fig. 2a,b).
Kaplan–Meier estimates and log-rank test to compare survival between stage T2N0M0 and stage T1N1M0 gastric cancer. a Gastric cancer-specific survival (GCSS) for the whole cohort; b overall survival (OS) for the whole cohort; c GCSS when examined lymph nodes were less than or equal to 15; d OS when examined lymph nodes were less than or equal to 15; e GCSS when examined lymph nodes were more than 15; f OS when examined lymph nodes were more than 15
To investigate the prognostic factors, the results of the GCSS and OS analysis using univariate and multivariate Cox proportional hazard regression models were further analyzed. For stage IB and T2N0M0 GC, in patients older than 70 years, the tumor location at the upper site, family income less than $60,000 and ELN ≤ 15 were significantly associated with poor GCSS and OS (Table 2, Supplement Table 1).
Marital status was only associated with poor OS (Supplement Table 3) but not with GCSS (Supplement Table 2) for stage T2N0M0 GC. For stage T1N1M0 GC, ELN ≤ 15, tumor location at the upper site, and lack of CT were significantly associated with the poor GCSS (Supplement Table 4) and OS (Supplement Table 5).
Effect of surgery alone versus adjuvant treatment on GCSS and OS in T2N0M0 group and T1N1M0 group
To clarify the effect of surgery alone (SA) or AT (including CT, adjuvant chemoradiotherapy (CRT), and RT) on prognosis, we compared the survival rate between SA group and AT group in T2N0M0 and T1N1M0 stages. In T2N0M0 stage: the AT group and the SA group demonstrated similar survival in GCSS (P = 0.241), but the SA group had a significantly worse OS rate than the AT group (P = 0.001) (Fig. 3a, b). In T1N1M0 stage: compared to the AT group, the SA group demonstrated a significantly worse GCSS rate (P < 0.001) and OS rate (P < 0.001) (Fig. 4a, b).
Gastric cancer-specific survival (GCSS) and Overall survival (OS) curves of surgery alone group (SA) and adjuvant treatment group (AT) for stage T2N0M0 gastric cancer (GC). a GCSS compared in SA and AT groups before propensity score matching; b OS compared in SA and AT groups before propensity score matching; c GCSS compared in SA and AT after propensity score matching; d OS compared in SA and AT groups after propensity score matching
Kaplan–Meier estimates and log-rank test comparing Gastric cancer-specific survival (GCSS) and Overall survival (OS) for stage T1N1M0 gastric cancer: surgery alone group (SA) vs. adjuvant treatment group (AT) and surgery alone group (SA) versus chemotherapy group (CT) versus chemoradiotherapy group (CRT). a GCSS compared in SA with AT; b OS compared in SA with AT; c GCSS compared in SA with CT and CRT; d OS compared in SA with CT and CRT
Effect of surgery alone versus chemotherapy versus chemoradiotherapy on GCSS and OS in T2N0M0 and T1N1M0
To further clarify the effect of different AT types on prognosis, we divided the patients into three groups (RT alone was excluded as the number of patients in this group was particularly small [16, 17]: SA group, CT group, and CRT group. For stage T2N0M0 GC: compared to the CT and the CRT group, the SA group demonstrated a comparable survival trend in GCSS (P = 0.183 for CT group, P = 0.578 for CRT group), but showed a significantly worse OS rate (P = 0.033 for CT group, P = 0.002 for CRT group). There was no significant difference between the CT group and the CRT group in GCSS (P = 0.360) and in OS (P = 0.954) (Supplement Figure 2A–2B). For stage T1N1M0 GC: compared to the CT and the CRT group, the SA group demonstrated a significantly worse GCSS rate (P = 0.006 for CT group, P < 0.001 for CRT group) and OS rate (P < 0.001 for CT group, P < 0.001 for CRT group). Among the CT and the CRT group, we did not observe significant difference in either GCSS (P = 0.958) or OS (P = 0.545) (Fig. 4c, d).
Comparison of survival after 1:1 matched between T2N0M0 group and T1N1M0 group
In the view of unmatched characteristics affecting the survival outcomes between T2N0M0 and T1N1M0 stages, we constructed a 1:1 (T2N0M0:T1N1M0) case–control matched analysis using the propensity score matching method. 772 patients for GCSS (Supplement Table 6) and 1098 patients for OS (Supplement Table 7) were included for further analysis. Based on the two matched groups, we found that matched T2N0M0 group had a better survival both in GCSS (P = 0.002) and OS (P = 0.009) than matched T1N1M0 group (Supplement Figure 1A–1B).
Comparison of survival after 1:1 matched in T2N0M0 group and T1N1M0 group according to the type of treatment
Considering that the unmatched AT type may affect the survival outcomes, we also constructed 1:1 (SA group:AT group) case–control matched analysis for T2N0M0 and T1N1M0.
For the matched T2N0M0 group, 654 patients for GCSS (Supplement Table 8) and 766 patients for OS (Supplement Table 9) were included. Based on the two matched groups, we found that matched surgery alone group (MSA group) had comparable survival both in GCSS (P = 0.471) and OS (P = 0.852) as matched adjuvant treatment group (MAT group) (Fig. 3c, d). For the matched T1N1M0 group, 268 patients for GCSS (Supplement Table 10) and 412 patients for OS (Supplement Table 11) were included. Based on the two matched groups, we found that the MSA group had a significantly worse survival both in GCSS (P < 0.001) and OS (P = 0.001) when compared to the MAT group (Supplement Figure 2C-2D).
Comparison of survival between T2N0M0 and T1N1M0 according to the number of examined lymph nodes
Group with ELN ≤ 15 included 763 patients for GCSS and 1198 patients for OS. Compared to the T1N1M0 group, T2N0M0 group exhibited a better survival both in GCSS (P = 0.003) and OS (P = 0.038) (Fig. 2c, d). Group with ELN > 15 included 554 patients for GCSS and 773 patients for OS. The T2N0M0 group demonstrated comparable survival both in GCSS (P = 0.146) and OS (P = 0.464) as the T1N1M0 group (Fig. 2e, 2f).
Comparison of survival after 1:1 matched between T2N0M0 and T1N1M0 according to the number of examined lymph nodes
Group with ELN ≤ 15 after matched included 430 patients for GCSS (Supplement Table 12) and 666 patients for OS (Supplement Table 13). Compared to T1N1M0, T2N0M0 had a better survival both in GCSS (P = 0.011) and OS (P = 0.030) (Supplement Figure 1C–1D). Group with ELN > 15 after matched included 338 patients for GCSS (Supplement Table 14) and 350 patients for OS (Supplement Table 15). Compared to T2N0M0, T1N1M0 demonstrated similar survival both in GCSS (P = 0.515) and OS (P = 0.270) (Supplement Fig. 1e, 1f).
Discussion
We sought to seek evidence to explore the differences between T2N0M0 and T1N1M0 GC, both classified as stage IB GC, using the SEER database. Overall, T2N0M0 group had the clinical pathological characteristics comparable to T1N1M0 group except the tumor size. The larger tumors were widely distributed in T2N0M0 group, while the smaller tumors were mainly aggregated in T1N1M0 group. These findings are consistent with the study of Du et al. [14].
The T2N0M0 group had a significantly better GCSS and OS than the T1N1M0 group, although more patients received AT in T1N1M0 group. The univariate and multivariate Cox proportional hazard regression models for the stage IB GC demonstrated that T1N1M0 was an independent risk factor of worse GCSS and OS for prognosis, which strongly supports the survival analysis results. Furthermore, following the propensity score matching, the T2N0M0 group was found to have a better GCSS and OS in comparison to the T1N1M0 group suggesting that T2N0M0 exhibited better survival than T1N1M0. These results are consistent with the findings of Gold et al. in SEER database from 1988 to 2008 [10]. Conversely, other reports demonstrated a comparable survival rate between the two subgroups [12,13,14,15]. According to the 8th edition AJCC GC staging system, it is widely accepted that the number of ELN should be at least 16 [18]. The majority of patients in Japan and Korea received extensive lymphadenectomies (D2 lymphadenectomy or greater) and had more ELN [19, 20]. However, most surgeons in the western countries perform more limited lymphadenectomies (D1 lymphadenectomy or less) because of high perioperative complications and mortality in D2 lymphadenectomy [21, 22]. The proportion of 16 or more ELN was only 39.22% after radical surgery in our study which is similar to the other study showing that more than half of the GC patients in the USA had a fewer than 16 ELN [23]. Furthermore, subgroup analysis revealed that T2N0M0 and T1N1M0 were consistent in survival when ELN were > 15, but T1N1M0 demonstrated a worse survival than T2N0M0 when ELN were ≤ 15. One of the obvious reasons to explain the survival difference between T2N0M0 and T1N1M0 is number of ELN which can cause stage migration [24, 25].
The AT strategies differed between T2N0M0 and T1N1M0 which may affect the survival. On one hand, AT demonstrated better survival than SA in GCSS but not in OS for T2N0M0. On the other hand, AT demonstrated significantly better survival than SA in both GCSS and OS for T1N1M0 which was consistent with the study of Jabo et al. [26]. Furthermore, AT was found to yield better GCSS and OS outcome than SA after case–control matched analysis for T1N1M0. However, AT demonstrated a better OS than SA for T2N0M0 before matched; this advantage was dismissed after matched. Therefore, T2N0M0 GC may not benefit from AT based on the similar GCSS and OS results after matched.
To further understand which specific treatment modalities might lead to the differences in survival, especially for stage T1N1M0 GC, AT was divided into two subgroups: CT and CRT. In the USA, CRT using 5-fluorouracil-based regimen is recommended after D1 gastrectomy for T1N1M0 GC. In Europe, CT is recommended after D2 gastrectomy for all stages except for T1N0M0 GC [27]. In contrast, the Japanese Gastric Cancer Treatment Guidelines recommend observation alone after curative resection for T1N1M0 and T2N0M0 GC [3]. Overall, there has been no consensus on the use of AT in T1N1M0 and T2N0M0 GC. In our study, CRT subgroup showed the same survival when compared to CT subgroup in both T2N0M0 and T1N1M0 GC. Similarly, Kim et al. reported the CT and CRT had the similar survival in T1N1M0 GC [28]. However, another SEER-based data showed that the CRT demonstrated a better survival in comparison to SA or CT in both T2N0M0 and T1N1M0 GC [29]. While their SEER results of this study were based on the 6th edition AJCC GC staging, our results were based on the 8th edition AJCC GC staging. Our results clearly showed that RT was not required after radical surgery in either the T2N0M0 or the T1N1M0 GC. The CT demonstrated a significantly better GCSS and OS than the SA for T1N1M0 GC. While lack of CT was not an independent risk factor affecting prognosis for stage IB and T2N0M0, it was an independent risk factor for T1N1M0 highlighting the profound influence of CT on the prognosis of T1N1M0 [30].
Although T2N0M0 and T1N1M0 are both stage IB according to the 8th edition AJCC classification, they have significantly different prognosis and treatment strategies, especially when ELN were ≤ 15. We should pay more attention to the improvement of surgical quality to obtain adequate lymph nodes to avoid stage migration.
Our study has limitations, based on the retrospective nature of the study. First, factors such as the specific surgical methods (D2 or D1), specific CT information (regimen and duration), lymphatic and/or blood vessel invasion, perineural invasion were not available in SEER database. Secondly, due to the inability to match 1: 1: 1 for lack of technology, we cannot match SA: CT: CRT with 1: 1: 1 using the propensity score matching. Thirdly, because all the patients included in our paper were surgery performed, we could only acquire the major clinical data and the TNM stage based on pathological results; so, our conclusions were only applied to pTNM stage but not clinical TNM stage (cTNM) or postneoadjuvant TNM stage (ypTNM). Moreover, the number of patients from the three subgroups would decline sharply after matched. However, based on our existing results, we can also explain the effects of specific AT methods on survival because of large sample size. Moreover, we only prove that T2N0M0 may not benefit from AT and that T1N1M0 requires CT. But in T2N0M0 patients with high-risk factors, AT may be necessary and in T1N1M0 patients with low-risk factors, CT may be avoided to prevent toxicity and discomfort. Finally, these findings need to be further validated in non-US cohorts.
In conclusion, stage T2N0M0 GC has a better survival than stage T1N1M0 GC when ELN were ≤ 15. After radical surgery, T2N0M0 GC may not benefit from AT, while T1N1M0 GC requires CT but may not require RT. However, more than 15 ELN are essential to avoid stage migration. A prospective and RCT is required for further confirmation of these results.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. https://doi.org/10.3322/caac.21388.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2016;14(10):1286–312.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20. https://doi.org/10.1056/NEJMoa072252.
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96. https://doi.org/10.1016/s1470-2045(14)70473-5.
Kwon OK, Kim SW, Chae HD, Ryu SW, Chung HY, Kim SW, et al. Validation of the 7th AJCC/UICC staging system for gastric cancer and a proposal for a new TNM system based on a prognostic score: a retrospective multicenter study. Ann Surg Treat Res. 2016;91(6):295–302. https://doi.org/10.4174/astr.2016.91.6.295.
Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012;214(1):88–96. https://doi.org/10.1016/j.jamcollsurg.2011.09.018.
Patel MI, Rhoads KF, Ma Y, Ford JM, Visser BC, Kunz PL, et al. Seventh edition (2010) of the AJCC/UICC staging system for gastric adenocarcinoma: is there room for improvement? Ann Surg Oncol. 2013;20(5):1631–8. https://doi.org/10.1245/s10434-012-2724-5jk.
Lin JX, Lin JP, Li P, Xie JW, Wang JB, Lu J, et al. Which staging system better predicts 10-year survival for gastric cancer? A study using an international multicenter database. Eur J Surg Oncol. 2018;44(8):1205–11. https://doi.org/10.1016/j.ejso.2018.05.014.
Gold JS, Al Natour RH, Saund MS, Yoon C, Sharma AM, Huang Q, et al. Population-based outcome of stage IA–IIA resected gastric adenocarcinoma: who should get adjuvant treatment? Ann Surg Oncol. 2013;20(7):2304–10. https://doi.org/10.1245/s10434-012-2852-y.
Aoyama T, Yoshikawa T, Fujikawa H, Hayashi T, Ogata T, Cho H, et al. Prognostic factors in stage IB gastric cancer. World J Gastroenterol. 2014;20(21):6580–5. https://doi.org/10.3748/wjg.v20.i21.6580.
Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116(24):5592–8. https://doi.org/10.1002/cncr.25550.
Du C, Zhou Y, Cai H, Zhao G, Fu H, Shi YQ, et al. Poor prognostic factors in patients with stage I gastric cancer according to the seventh edition TNM classification: a comparative analysis of three subgroups. J Surg Oncol. 2012;105(3):323–8. https://doi.org/10.1002/jso.22082.
Lu J, Cao LL, Li P, Xie JW, Wang JB, Lin JX, et al. Significance of preoperative systemic immune score for stage I gastric cancer patients. Gastroenterol Res Pract. 2018;2018:3249436. https://doi.org/10.1155/2018/3249436.
Datta J, McMillan MT, Shang EK, Mamtani R, Lewis RS Jr, Kelz RR, et al. Omission of adjuvant therapy after gastric cancer resection: development of a validated risk model. J Natl Compr Cancer Netw. 2015;13(5):531–41.
Stessin AM, Sherr DL. Demographic disparities in patterns of care and survival outcomes for patients with resected gastric adenocarcinoma. Cancer Epidemiol Biomark Prev. 2011;20(2):223–33. https://doi.org/10.1158/1055-9965.epi-10-0158.
Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20(2):217–25. https://doi.org/10.1007/s10120-016-0601-9.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition—response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer. 2001;4(1):1–8.
Yoon SS, Yang HK. Lymphadenectomy for gastric adenocarcinoma: should west meet east? Oncologist. 2009;14(9):871–82. https://doi.org/10.1634/theoncologist.2009-0070.
Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347(9007):995–9.
Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345(8952):745–8.
Arsoniadis EG, Marmor S, Diep GK, Hui JYC, Jensen EH, Tuttle TM, et al. Survival rates for patients with resected gastric adenocarcinoma finally have increased in the United States. Ann Surg Oncol. 2017;24(11):3361–7. https://doi.org/10.1245/s10434-017-5992-2.
Yang ZL, Zhu MH, Shi Q, Lu FM, Wang CX. Prognostic value of the number of lymph nodes examined in patients with node-negative gastric cancer. J Gastrointest Surg. 2018. https://doi.org/10.1007/s11605-018-3947-y.
Yuan SQ, Chen YT, Huang ZP. Equipping the 8th edition American Joint Committee on Cancer Staging for Gastric Cancer with the 15-node minimum: a population-based study using recursive partitioning analysis. J Gastrointest Surg. 2017;21(10):1591–8. https://doi.org/10.1007/s11605-017-3504-0.
Jabo B, Selleck MJ, Morgan JW, Lum SS, Bahjri KA, Aljehani M, et al. Comparison of perioperative chemotherapy with adjuvant chemoradiotherapy for resectable gastric cancer: findings from a population-based study. J Gastrointest Oncol. 2018;9(1):35–45. https://doi.org/10.21037/jgo.2017.10.13.
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57–63. https://doi.org/10.1093/annonc/mdt344.
Kim SM, An JY, Lee J, Sohn TS, Kim S. Adjuvant chemotherapy versus chemoradiotherapy versus surgery alone for early gastric cancer with one or two lymph node metastasis. Ann Surg Oncol. 2018;25(6):1616–24. https://doi.org/10.1245/s10434-018-6434-5.
Park JH, Ryu MH, Kim HJ, Ryoo BY, Yoo C, Park I, et al. Risk factors for selection of patients at high risk of recurrence or death after complete surgical resection in stage I gastric cancer. Gastric Cancer. 2016;19(1):226–33. https://doi.org/10.1007/s10120-015-0464-5.
Moon JH, Fujiwara Y, Hirao M, Imamura H, Kimura Y, Fujitani K, et al. Randomized controlled trial of adjuvant chemotherapy with fluoropyrimidines versus surgery-alone for gastric cancer. Anticancer Res. 2017;37(6):3061–7. https://doi.org/10.21873/anticanres.11661.
Funding
Liaoning provincial special fund for central guiding local science and technology development plan 2018 (2018416017).
Author information
Authors and Affiliations
Contributions
Wang Y performed the majority of experiments and analyzed the data and drafted the manuscript; Zheng ZZ, Zhao Y designed the research; Zhang J, Guo S, Dong Z, Xiang-yu Meng, Guo-liang Zheng, Dong Yang contributed reagents/materials/analysis tools; Zhao Y provided critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest related to this study.
Ethical approval
The contents of this article are data mining from the Surveillance, Epidemiology and End Results (SEER) database. The SEER database is open and shared. The data released from the SEER database did not require informed patient consent because cancer is a reportable disease in every state in the US.
Informed consent
The data released from the SEER database did not require informed patient consent because cancer is a reportable disease in every state in the US. Therefore, we do not need to sign further informed consent in our article (not applicable).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Y., Zhang, J., Guo, S. et al. Implication of lymph node staging in migration and different treatment strategies for stage T2N0M0 and T1N1M0 resected gastric cancer: a SEER population analysis. Clin Transl Oncol 21, 1499–1509 (2019). https://doi.org/10.1007/s12094-019-02078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02078-y