Abstract
The gastrointestinal system, also referred to as the gut, is a universe that colonizes trillions of microbes. In addition to its digestive functions, the gut represents a biosystem that determines all the health vectors. It is now recognized as one of the body's defense systems, and good gut health regulates the body’s immune responses. Disturbance of this barrier can trigger many diseases, including respiratory tract infections, as there is a close correlation between the gut microbiome and the chances of triggering illness. This review investigates the various factors affecting the gut microbiome, the diseases that can result from the dysregulation of the same, and their molecular mechanisms. The most basic solution to tackle this problem is to maintain the gut microbiome at the desired level. Timely diagnosis and interventions are needed for the proper management of the ensuing conditions. It is important to address the effects of factors on the gut microbiome and thereby regulate this level. The study also found that dysregulation in the system can lead to various diseases such as asthma, COPD, lung cancer following their respective pathways. In short, this paper reinforces the importance of the gut microbiome, the need to maintain its average level, and the need for proper interventions to treat the consequences. The manuscript posit that medications, diet as well and good physiological conditions of the human body can alter the microbiome and can ward off respiratory infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A microbiome refers to a group of microorganisms that live in a given habitat and interact with one another. According to estimates, the human gut contains approximately 200 different bacteria, viruses, and fungi, all of which contribute to the body's metabolic activities and the maintenance of good health [1]. The bulk of gut microbiome are not pathogenic in nature and live in a symbiotic relationship with enterocytes, and the immune system has evolved to co-exist with the healthy microbiota while fighting to invade pathogenic microbes. A healthy individual generally houses over 1000 species of microbiota, mainly belonging to the phyla Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, Verrucomicrobia and Arachaea. Bacteroidetes and Firmicutes are the chief forms, whereas Cyanobacteria, Fusobacteria and Spirochaeatae comprise lesser forms of bacteria present in the gut. The MetaHIT (META genomics of the Human Intestinal Tract) Consortium proposes a method of categorizing gut flora depending on species diversity, which groups them into balanced symbiotic host-microbial forms that are stable across regions and genders but retort variably to food and medicines. These groups were labelled as enterotypes, and there were three enterotypes. Enterotype 1 has a larger portion of Bacteroides; Enterotype 2 has a predominance of Prevotella, and Enterotype 3 has a dominance of Ruminococcus. The major shortcoming of this enterotyping notion is that it does not explain the proportional distribution of various organism types within people [2]. Many common microorganisms that reside in the human digestive tract (GI) have specific metabolic activities and are crucial for the maintenance of health and the prevention of illness. In medicine, one of the extensively debated issues is the relationship between gut microbiome activity and human health.
Asthma, COPD, and other respiratory viral infections are frequently accompanied by gastrointestinal (GI) symptoms, including nausea, vomiting, abdominal pain, and diarrhea. Patients with gastrointestinal diseases are more likely to develop respiratory ailments, such as inflammatory bowel disease (IBD) and gastroesophageal reflux disease (GERD), than those who do not have these conditions (GERD) [3] These connections suggest a critical point of interaction between the stomach and the lungs. The human microbiome is considered to maintain homeostasis, the develop illness, and transmit information across mucosal locations. Alterations in microbial communities or diversification have an influence not only on the organs that have been colonized but also on other organs and systems Dysbiosis of the gut microbiota has been linked to a variety of disorders, including allergies, autoimmune diseases, diabetes, obesity, and cancer [4].
It is impossible to emphasize the importance of a well-balanced microbial community in the gut for immune function and overall health. Several studies have revealed that the gut microbiota can impact pulmonary immunity through the gut–lung axis, which is a critical cross-talk between the gut microbiota and the lungs When endotoxins, microbe metabolites and cytokines (IL-6-, IL-1, TNF-α), pass-through this axis, they enter the circulation and when inflammation occurs in the lungs, the lung-gut axis can produce changes in the blood and gut flora, which can be harmful [4].
These findings illustrate the significance of gut microbiota in the human body, their relationship with health, as well as respiratory illnesses, including asthma and COPD, infectious diseases, and lung cancer. They also demonstrate current advances and future prospects. More studies into the relationship between gut microbiota and human health are needed, as this report points out in its conclusion.
Fundamental Functions of Gut Microbiota
Good microbiomes contribute to metabolic, antipathogenic and immunomodulatory properties. They play a significant role in food and mineral absorption, enzyme production, synthesis of vitamins and amino acids, and biosynthesis of short-chain fatty acids (SCFAs). Gut microbiomes have an impact on the majority of human physiological processes, both in healthy and sick states, through the performance of these four fundamental tasks. The fermented polysaccharides of Bacteroides, Roseburia, Bifidobacterium, Faecalibacterium and enterobacteria lead to the formation of SCFAs such as acetate propionate and butyrate [5,6,7,8,9]. Butyrate acts as a source of energy for human colonocytes, promotes death in colon cancer cells, increases intestinal glucogenesis, maintains an oxygen balance in the intestines and prevents disruptive intestinal flora. Propionate regulates gluconeogenesis and the signalization of satiety through interactions with fatty acid receptors in the stomach. The most common SCFA, acetate, is crucial to the growth of other bacteria. In cholesterol and lipogenesis metabolic processes, as well as central appetite regulation, it can have a role [2]. Bacteria in the intestines also play a critical role in the creation of vitamin K and a variety of vitamin B components. These Bacteroides strains have been reported to produce linoleic conjugate acid (CLA), which is known to be immunomodulatory, antidiabetic and hypolipidemic [5].
C-type lectins, (pro)defensins and cathelicidins have been shown to be produced by host Paneth cells in response to the presence of the gut microbiota using pattern recognition receptor (PRR). PRR are proteins with the ability to recognize molecules repeatedly found in pathogens. As Lactobacillus spp can produce lactic acid, it can increase the host lysozyme's antimicrobial activity by breaking down the bacteria's cell wall. This is yet another illustration of the gut microbiota's antibacterial properties [5].
In addition, they aid in the preservation of the intestinal barrier and the gastrointestinal system's architecture. Bacteroides thetaiotaomicron was discovered to enhance the synthesis of the desmosome nutrition-critical short proline-rich protein 2A (sprr2A). For protection of the digestive tract against cytokine-induced cell death via the epithelial growth factor receptor (EGFR) and protein kinase C pathways, Lactobacillus rhamnoSus GG bacteria produce two soluble proteins (p40 and p75) (PKC). Angiogenin-3 is produced by the intestinal microbiota and serves as a transcription factor for gut mucosal structural stability [5].
Gut microbiota play a critical role in the metabolism of xenobiotics, which could have substantial implications for the development of new treatments for a wide range of disorders [5].
Factors Affecting Gut Flora
The intensity of colonization and the makeup of the resident microbial communities varied significantly among anatomical locations. It is determined by transit rates, host secretions, ambient factors, substrate availability, and gut wall organization. As a result, the stomach and proximal small intestine contain a small number of microbiota that can withstand the pH levels, oxygen exposure, and relatively fast transit rates that predominate in these areas. On the other hand, conditions in the large intestine typically encourage the formation of an extraordinarily dense microbial population dominated by obligate anaerobic bacteria. Individual factors affecting microbial composition depend on the factors prescribed in Fig. 1 [10]. Although the type of feed has significant influence on the early stages of development of the microbiota, following principal inoculation, the time-related change is controlled by dietary habits, lifestyle, life events, and ecological variables such as antibiotic usage [5]. There are numerous limitations in the design and interpretation of human microbiome research. Human studies often provide faecal samples reflecting the distal colon microbiota but do not enable access to the caecum and proximal colon bacteria. Furthermore, human studies in this sector often involve a small number of participants, and the participants' dietary habits and lifestyles vary greatly. Typically, faecal samples are obtained at a single point in time to represent the short-term rather than long-term influence of food, and the overall number of bacteria is not generally evaluate [10].
Birth Method
According to a number of research, the sequence of gut microbiota formation differs from that which occurs with a vaginal birth. Because labor triggers immune responses in the uterus that do not exist after a caesarean section, the delivery method has an impact on the immunological milieu for newborns. TNF-α, interleukin-1, interleukin 6, interleukin 8, and other inflammatory cytokines increase over time. A rise in the neonate's blood leukocyte count suggests that proinflammatory cytokines produced in the uterine environment are responsible for activating the foetal immune system while the mother is pregnant. Cord blood monocytes produced less IL-6 following cesarean section delivery. Neonatal immunological cytokines and cytokine receptors were found in the serum of C-section baby C-section babies. On the first day of life, infants born via caesarean section had decreased IFN-, IL-6-, IL-1, TNF-, and soluble TNF-RI levels in their umbilical cord blood compared to those born via vaginal delivery.
Aagaard and coworkers used innovative sequencing for the characterization of a distinctive microbiome in the human placenta, which sparked a new study field and shed light on microbial populations in the fetal domain, challenging the sterile womb prototype. The function of these microbial communities in commencing the creation of the human microbiota through in utero dissemination and altering human health was a critical topic. Concerns were soon expressed, pointing out, amid other shortcomings, that DNA identification did not give affirmation for live bacteria, rendering these findings as insufficient to question womb sterility [11].
Rackaityte et al. reignited the debate by providing proof for the existence of DNA in bacteria and the bacteria of the intestinal region of the foetus. They relyed on 16S rRNA sequencing gene, qPCR, microscopy, and data of culture. This study has now been called into question because the re-analyzed sequence data do not agree with the primary sequence data [11, 12].
The first microbial inocula of the infant are substantially differentiated in each case. The newborns delivered via caesareans are not exposed to maternal vaginal and faecal microbes. Instead they are colonised with skin bacilli, most likely from the surrounding environment. Although most vaginal and skin bacteria do not appear to colonise the newborn intestines, their presence may have a distinct influence on the colonisation potential of others [11]. Lactobacillus and Prévotella are two organisms that are the best instances of this colonisation, which colonise the intestines of born children in the vagina. In contrast, following delivery, the mother's microbiome predominates in the baby's intestines, as shown by Streptococcus, Corynebacterium, and Propionibacterium in the newborn's stomach. During development, preterm bacteria are supposed to maintain the gut-associated lymphoid tissue (GALT) and contribute to the production of innate immunity [5].
Feeding Method
Breastfeeding is a significant element affecting the development of a child's gut microbiota, followed by the proportion of carbohydrates, protein, and fat in a child's dietary composition. In formula-fed babies, the gut niche is dominated by Enterococcus, Enterobacteria, Bacteroides, Clostridia, and other anaerobic Streptococcus, while the gut niche is dominated by Bifidobacteria and Lactobacillus in breastfed babies [5]. A total of 600 bacterial species, including several beneficial Bifidobacterium genera, have been identified in breast milk.
Human breast milk contains oligosaccharides, also known as human milk oligosaccharides (HMOs), which are one of the most abundant solid elements in human milk but lacking in most formula nutrition and are thought to play a key role in the gut microbiota distinctions between breastfed and formula-fed infants. They are indigestible and do not lend energy to the newborn, but they function as prebiotics, which are precursors for intestinal microbe fermentation processes, boosting good gut microorganisms such as Bifidobacteria, which may inhibit diarrhoea and microbial infection until the suckling period. Breastfed infants had more Bifidobacterium in their faeces than formula-fed infants, and the greater relative percentage of acetate in breastfed infants compared to formula-fed infants may be related to the lack of HMO in formula. Immunological support is provided by Bacteriodetes proliferation, which raises immunoglobulin levels which alter the intestinal immune system and enhance the intestinal mucus system in order to avoid dangerous bacterial activities [9].
Secretory IgA (sIgA) also plays a role in the creation of the neonatal gut flora. Because infants generate only trace amounts of sIgA, breastmilk-derived sIgA prevents harmful germs from expanding and penetrating the infant's intestinal immune system. Experiments in mice with IgA-deficient dams showed that breastmilk-derived sIgA has a long-lasting function in moulding the gut flora. In the absence of sIgA from breastfeeding, the family Lachnospiraceae was elevated. Milk sIgA is also required for the coating of proinflammatory segmented filamentous bacteria (SFB) [13].
Dietary Patterns
Even at maturity, food is the most critical factor in determining the composition, diversity, and quality of the microbiota. As a general rule, increasing the amount of dietary soluble and fermentable fibre that is consumed (such as fruits and vegetables) is correlated with greater diversification and variety of the gut microbiota Individuals who consume this diet have a higher prevalence of Firmicutes phylum insoluble carbohydrate metabolising bacteria, such as Ruminococcusbromii, Roseburia, and Eubacterium rectale [5]. In addition to being a source of active resources, seaweeds include bioactive components that have a lot of beneficial qualities, like antibiotic, anti-inflammatory, anticoagulant, antiviral, and apoptotic capabilities. Gelidium seaweed supplementation substantially enhanced the activity of Bifidobacterium genera in humans but had no influence on the expression of other Bifidobacterium genera. In addition, there has been an increase in the production of SCFAs. Food additives, including sweeteners with a high concentration of sucrose and emulsifiers that are often found in packaged food, have been demonstrated to influence the human gut flora [5]. A cohort study that looked at the relationship between dietary variables and the microbiome found that consuming legumes, grains, seafood, and nuts is linked to a drop of opportunistic bacteria clusters, endo toxogenesis, and inflammatory markers in faeces. Conversely, they found that eating nuts, fruits, oily fish vegetables, and grains increased the abundance of commensals such Roseburia, Eubacterium and Faecalibacterium spp [14].
Some of the dietary elements affecting the diversity of gut microbiota are given in Table 1.
Age
Transitions in gut microbiota have been documented in people of all ages, including newborns, toddlers, adults, and the elderly [6]. Even in pregnancy, microbes may invade the baby gut. According to research, the first meconium is rich in bacteria from the species Escherichia, Enterococcus, Leuconostoc, Lactococcus, and Streptococcus. Bifidobacterium and Lactobacillus are bacteria that colonise the intestine in pre-term babies, and they vary depending on the kind of feeding patterns [5]. Actinobacteria is more prevalent during weaning, declines after weaning, and continues to decrease with age. Firmicutes is more common in children over the age of four than in children under the age of four. Proteobacteria are more prevalent in the elderly (those above the age of 70). Bifidobacterium, which down regulates pro-inflammatory signals, decreases as people age [6].
Age-related dysbiosis in humans is attributed to a reduction in Clostridiales and Bifidobacterium, an increase in Proteobacteria, and a predominance of pathobionts such as Enterobacteriaceae. Upregulation of pro-inflammatory interleukins (IL-6 and IL-8) in elderly people tend to be linked with a decline in the health-aiding SCFA-synthesizing Lachnospiraceae and an elevation in the colitis-promoting Erysipelotrichaceae, implying that an amended immune response gives rise to microbiome modifications that are far more counterproductive to the host. A decrease in mucin production alters the relative makeup of the mucin-metabolizing taxa in the SCFA-producing Clostridiaceae, Bacteroidaceae, and probiotic neurotransmitter-producing Bifidobacteriaceae families, causing cognitive impairment in the host [14].
Medications
A substantial amount of data now clearly shows that antibiotic usage has numerous immediate and long-term consequences in the normal gut microbiota ecosystem. The capacity to induce competitive exclusion is one of the most important characteristics of healthy gut microbiota against pathogens. Antibiotics were shown to impair the competitive exclusion mechanism, resulting in Salmonella infection soon after antibiotic treatment [5]. Broad-spectrum antibiotics, such as clindamycin, have been shown to have the longest-lasting impacts on the makeup of the gut microbiota in babies and young children. Neonatal antibiotic exposure may cause microbial dysbiosis, which may be a risk factor for inflammatory bowel disease [19]. Many nonantibiotic medications, including those used to treat type 2 diabetes, have been shown to have an effect on gut flora. Recent human research verified metformin's impact on gut flora [20]. Several research have been conducted to study the impact of frequently used medicines on the makeup and metabolic activity of the gut microbiota. The use of opioids, paracetamol, or proton pump inhibitors is linked to an increase in the richness of taxa from the families Streptococcaceae, Gemellaceae, Peptostreptococcaceae, and Fusobacteriaceae, as well as a decrease in the richness of taxa that produce SCFAs, such as Roseburia and Eubacterium rectale [21].
Exercise
There seems to be variance in the makeup of gut microbiota depending on active vs sedentary lifestyle. Active women had a greater number of bacterial species that are beneficial to health, such as Faecalibacterium prausnitzii, Roseburia hominis, and Akkermansia muciniphila; inactive women had higher abundances of Paraprevotella and Desulfovibrionacea. Obese teenagers who were placed on a calorie restriction with increased physical activity had an increase in Bacteroidetes and a reduction in Clostridia [6].
Gene Interactions and Gut Immunity
The quantity of particular bacteria present in the gut microbiota is controlled in part by the host's genetic composition, which affects host metabolism and, ultimately, health. Family members have more comparable microbial communities than unrelated people, and monozygotic twins had identical gut microbiota than dizygotic twins [19].
Mutualist bacteria found in vertebrates support critical immunological forbearance and modulatory mechanisms in the organs. These include non-specific immune response in the skin, the respiratory tract's response to influenza, and the intestinal formation of gut-associated lymphoid tissue (GALT) and regulatory T cells. Elimination or alteration of these microorganisms impairs human immunity or can result in auto-immune diseases [22].
Additionally, commensal bacteria have been demonstrated to have a role in coordinating and training adaptive immunity as well as in the growth and homeostatic mechanism of innate immune cells [23]. On the other hand, numerous immunological processes, including physical barriers (i.e., host-secreted mucus layers; and compounds for preventing microbial growth, recognition molecules and associated signalling, responses in the effector, all contribute to microbiota composition regulation (i.e., secretion of antibodies) [24]. Progress in transcriptomics (RNAseq) has resulted in a greater knowledge ofhost role in different systems, including immunity and immune response. These developments may enable the growth of research into bidirectional communication [25, 26].
Beyond current experimental models with simplistic microbial constitutions, interactivity of microbiome and host (here, “microbe-immune feedbacks”) are required. Despite these technological advancements, RNAseq has not been widely used to the study of microbe-immune feedbacks, specifically in complex habitats. Not many studies have discovered an association between the content of the microbiome and the expression of immune genes [27].
The majority of these studies, however, show the importance of a more basic microbiome or even monocultures. Although these correlations were limited to the constricted colonic microbiome, one study looked into more complicated relationships and found robust links between gene expression and microbiome content in colonic epithelial cells [28]. Studies investigating the feedback relationship between microbiome composition and host gene expression, in particular in gut epithelial tissue, have largely focused on localized gene expression. It's because of this that we know so little about the systemic effects of microbiota composition on immune-relevant distant gene expression [29].
Compounds Showing Gut Microbiome Dysbiosis
Humans are exposed to a variety of chemicals on a daily basis that can influence the gut microbiome causing dysbiosis. Evidences show that chemical exposure can have a significant effect on both the human body as well as the microbiome. Most of these effects are a result of the systemic effects of the pathologic changes happening in the body with the process. One of the evidences is based on the studies condcuted by Zhan et al. Showed thart exposure to 2,3,7,8-Tetrachlorodibenzodioxin interfered in the distribution of gut microbiome. To elaborate, the gut microbiome dysbiosis can be due to metabolic disorders, phenotypic changes etc. The vulnerable factor is that there are less chances for the gut microbiome to recover after the chemicalexposure. The following table provides the name and structure of compounds responsible for gut microbiome dysbiosis and in respiratory diseases (Table 2) [30, 31].
Respiratory Diseases and Gut Microbiota
Current theories are illuminating a relationship between gut microbiome and lung immunity. The fact that their mucosal tracts have identical genesis and features of physiology and structure, such as direct contact with the mouth and pharynx, physical barrier with projections of microvilli and cilia, as well as secretory IgA and mucus-producing goblet cells, may contribute to their close relationship. As a result, the impacts of the gut microbiota and their metabolites affect the immune response in distal mucosal locations like the lungs [32].
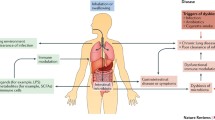
The regulation of the populations of extraintestinal T cells, development of oral immunological tolerance with Tregs, the production of SCFAs and systemic inflammation are all possible mechanisms connecting intestinal microbiota with respiratory diseases. Blood and lymphatic systems with control of the immune and inflammatory reactions in the lungs may have immune cells and cytokines, and the bioactive components of gut flora such as SCFAs, which further impact respiratory health and illness [33]. Some of the factors initiating respiratory diseases via gut dysbiosis are given in Fig. 2.
Asthma
Asthma is a chronic inflammatory disease induced by abnormal immune responses in susceptible people to common environmental antigens. The continual increase in asthma prevalence in industrialized countries cannot be ascribed only to hereditary causes, indicating that certain environmental variables associated with contemporary living promote asthma. The pathophysiology of asthma may be linked to variables like caesarean birth, antibiotic usage during the newborn period, a low-fibre maternal diet, formula feeding, and exposure to a range of microorganisms in the environment. Dysbiosis, or microbial imbalance, particularly in the gut microbiota, has been linked to the onset of a variety of illnesses, including allergy diseases and asthma [34].
Numerous human epidemiological research on the relationship between gut microbiota composition and allergy/asthma have been performed over the last 15 years. The overwhelming majority of this research indicates that individuals with allergies and asthma had altered amounts of “beneficial” and possibly dangerous bacteria as compared to healthy ones.
According to the “hygiene hypothesis,” early-life subjection to certain microbiota components is critical for immune system establishment and maturation, and their deprivation causes vulnerability to asthma and allergy disorders [33]. The microbiota of asthmatics included fewer microbial organisms than the microbiome of healthy people. In instances, Faecalibacterium prausnitzii, Sutterella wadsworthensis, and Bacteroides stercoris were reduced, while Clostridium with Eggerthellalenta was over-represented. According to functional analysis, the SCFAs in the microbiome of asthma patients may be changed.
In a study, it was discovered that the amount of fermentable fibre in the diet altered the makeup of the gut and lung microbiota, precisely the ratio of Firmicutes to Bacteroidetes.. Increasing data suggests that the establishment, development, and modulation of the immune system is dependent on microbial colonization of mucosal tissues during infancy. At least in part, the gut microbiota's impact on asthma can be attributed to bacterial metabolites, which may influence immunological responses in other parts of the body. In humans, SCFAs are the most well-known anti-inflammatory metabolites. An increased level of butyrate and propionate in a child's stool reduces his or her risk of developing asthma during the early stages of childhood [34].
Researchers looked into the early years of gut microbiota development in children with asthma risk and how Lactobacillus supplementation in childhood might affect it. Collecting samples from healthy newborns and high-risk babies who were given daily oral Lactobacillus rhamnosus or a placebo for six months. Human Research Program participants have a distinct meconium microbiota, which is primarily glycolytic and lacking in diversity of anti-inflammatory lipids at six months of life. It was found that infants who were given daily oral Lactobacillus rhamnosus were partially protected from these defects, but the effect was lost at the age of 12 months, six months after supplementation had been withdrawn, indicating the distinct but malleable development of intestinal flora in HR infants and offering novel practice in early life for early prevention [35, 36].
Adding probiotics to your diet may improve your immune system. Clinical research is needed to learn more about the impacts of asthma. In a study by Zhang et al. 6–8 week old male Balb/c mice were sensitised intraperitoneally with ovalbumin. Three different doses of probiotic suspension was administered. Intervention with a mixed strain increased Lactobacillus genus levels while decreasing ovalbumin-induced allergic airway inflammation. These findings offer new evidence for the use of probiotics in allergic diseases and support the notion that targeting gut microbiota will be an effective treatment for allergic airway diseases [37].
Providing immune training to the fetus by maternal environment open up possibilities for asthma prevention after delivery. The hygiene idea suggests that encouraging breastfeeding and increasing exposure to other microorganisms are effective preventive measures during the postnatal period. During pregnancy, the immune system can be influenced by maternal diet and nutritional supplements, which can have long-term effects on a child's ability to breathe easily. Pregnancy-induced alterations in the microbiota, including an increase in the number and activity of T-regulatory cells, have been linked to a high-fiber diet. Lactobacillus rhamnosus, a postnatal probiotic, was found to reduce the incidence of childhood asthma, most likely because probiotics can alter the levels of SCFAs and alter the composition of the microbiome [38].
In a study of HDM-induced allergic asthma in mice, researchers observed that two Lactobacillus strains reduced airduct inflammation, repressed Th2 and Th17 immune responses, and promoted Treg responses, while also modifying the gut microbiome heterogeneity, composition, and metabolism. This is a critical technique for lowering inflammation in the respiratory tract. There are two new strains of Lactobacillus reuteri (L. reuteri CCFM1072 and L. reuteri CCFM1040) found in this study, which could be used to improve allergic asthma prevention and treatment, as well as to better understand how Lactobacillus affects allergic asthma symptoms by influencing the microbiota in the gut [39]. We believe that having a well-balanced gut microbiota population is advantageous in terms of health, while a lack of diversity can lead to latent pathological illnesses.
Mechanism at Molecular Level
Th2 cells, type 2 B cells, eosinophils and mast cells, and "signature" type 2 mediators such as IL-4, IL-5 and IL-13 are all involved in allergic asthma, or exercise-induced asthma. The inflammatory response to allergens and exercise is triggered by Th2 cells. Th1 and Th17 cells and neutrophils infiltrate in obesity-related asthma, neutrophilic asthma, and paucigranulocytic asthma, as well as type I interferons, the NLRP3 inflammasome, and IL-1b and IL-17 signatures in the inflammation [35] (Fig. 2).
COPD
COPD, which is prevalent, preventable, and curable, has an established respiratory dysfunction that is caused by airflow restriction and increased airway inflammation. Because of its frequency, morbidity, and death, COPD has been a significant public health issue worldwide. While an abundance of research has established the fact that COPD patients often deal with chronic gastrointestinal illnesses such as inflammatory bowel disease (IBD), there is only a limited amount of research that has looked at the microbiome in COPD patients (Fig. 3).
There has been much research demonstrating that the primary cause of cigarette smoking is the development of COPD. According to the findings reported by Alqahtan et al., the gut microbiota changes as a result of increased cigarette smoking. In comparison to nonsmokers, smokers have a different gut microbiome, according to the findings. Following that, they observed a rise in the concentrations of Actinobacteria and Firmicutes, as well as a reduction in the concentrations of Bacteroidetes and Proteobacteria. The family Lachnospiraceae spp. was discovered in the mouse microbiome. Smoke exposure in the stomach exacerbated it. It has been established that the gut microbiota may have a role in COPD development (which is caused by cigarette smoking) [40] (Fig. 4).
Specific Molecular Mechanism for COPD
Asthma and COPD are both respiratory disorders that cause airway obstruction and persistent inflammation. Even so, the nature and sites of inflammation vary across various illnesses, resulting in pathophysiology, clinical symptoms, and treatment response that are all distinct from one another. The differences in inflammatory cells and the profile of inflammatory mediators are highlighted in this study, which analyses the inflammatory and cellular processes of asthma and COPD [41]. These differences are responsible for the clinical presentation of COPD andasthma, as well as their response to treatment. Despite the fact that COPD and asthma are typically different diseases, some persons have characteristics in common that might be attributable to the occurrence of two typical diseases at the same time or two well defined phenotypes of each disease. It is critical to have an understanding of the fundamental cellular and molecular principles of asthma and COPD in order to develop novel therapies in areas where there is a pressing need, such as severe asthma, efficacious asthma treatment, and effectual antiinflammatory medications for COPD (Fig. 5).
Alqahtan et al. have looked at probiotics and COPD; nevertheless, those that have shown a link between the gut microbiota and COPD are worth noting [40]. When rats with chronic obstructive pulmonary disease (COPD) were given probiotics including Lactobacillus rhamnosus and Bifidobacterium breve, their inflammation and lung damage were both lessened. A study looked at the similarities between alterations in the lung and gut microbiota caused by smoking and dietary fibre. Gut microbial metabolism of dietary fiber to SCFAs tends to reduce lung inflammation and immune cell activation. Thus they concluded that the impacts of a low-fiber diet and smoking exposure on gut microbiota are comparable, and microbiota may be a probable internal mechanism that might both prevent and treat cigarette smoke-induced COPD [41].
Additional research by Walter examined the effects of probiotics on COPD and offer novel treatment approaches that are needed in the future [42].
Lung Cancer
Gut microbiota is used as a therapeutic tactic used to minimize the toxicity of lung cancer and related disorders lung disorder. The lungs are usually not sterile or free from any given bacteria [43]. The composition of the microbiome is determined by the rules in the ecological setup. The composition is felt greater than in the gastrointestinal tract, according to Makki et al. [44].
Microorganisms are known to cause carcinogenic potential that has rapidly been evolving so that the lung has a distinct microbiome within it. Somatic mutations, which originate within cells and multiply when exposed to cancer agents, have been found to be the most common cause of cancer in cancer patients. Somatic cell proliferation can increase the risk of infection in cancer patients who eat foods that speed up and multiply these cells.
Dysbiotic microbiota may have a role in the growth and maintenance of an inflammatory milieu, which is considered to be responsible for up to 20% of all malignancies. An inflammatory milieu may play a role in carcinogenesis in the digestive system, for example, according to certain studies. Membrane receptors allow human cells to exchange information with the outside world, while signal transduction allows them to respond to the information they receive. In spite of the complexity of this process, the cell's ability to adapt to a changing external environment is the primary goal. The immune system is typically able to develop an effective defense when faced with a microbial invasion. Corresponding microenvironmental changes may be detrimental to patients with long-term illnesses [45, 46]. Membrane receptors such as pattern recognition receptors (PRR), cluster of differentiation (CD), and toll-like receptors (TLR) proteins, among others, recognize microbes, microbial products, pro-inflammatory cytokines, signaling molecules, and changed human proteins and nucleic acids. Extracellular molecular signals, such as those associated with apoptosis, cell cycle regulation, and cell proliferation, can all have an impact on these processes. Oncogenes, reactive oxygen species, and cellular repair mechanisms can all lead to the development of mutations in the body. Mutant cells that survive, are chosen, and proliferate can lead to carcinogenesis [45, 46]. Microbiome-induced immunity against lung cancer may be linked to differences in lung and gut microbiome makeup, which calls for more exploration.
Genotoxicity has been linked to a slew of bacteria. Double-stranded DNA damage can be caused by the Bacteroides fragilis toxins. Chromosome instability can be exacerbated by bacteria-produced chemicals, such as superoxide dismutase [47]. The results previously presented by Greathouse et al. also shows a connection between Acidovorax and tumors harbouring TP53 mutations. If this link is causative, we don’t know yet (Table 3).
Specific Molecular Mechanism for Lung Cancer
In Western countries, non-small cell lung cancer (NSCLC) is the prevailing cause of mortality from malignant disease. Improved clinical results will result from greater knowledge of the molecular mechanisms underpinning NSCLC etiology, pathophysiology, and treatments. Many researches has discovered possible biomarkers for NSCLC that could be used in the diagnosis, screening, and monitoring of treatment success. Finally, research into the molecular mechanisms of NSCLC growth and the molecular mechanisms of action of currently utilized cytotoxic medicines has progressed [50]. This could help with the development of new treatments and the discovery of new targets. Taken together, these advancements point to a better knowledge of NSCLC’s molecular biology and treatment, which could lead to better results for this fatal illness (Fig. 6).
Respiratory Infectious Diseases
Coronavirus 2019 (COVID-19) was more than just an outbreak; it was caused by SARS-CoV-2, the coronavirus that causes severe acute respiratory syndrome. Anal swabs and stool samples from 50 percent of COVID-19 patients contained SARS-CoV-2 virus, indicating an extrapulmonary site for viral reproduction and activity [51]. According to the results of the study, some COVID-19 patients had lower levels than healthy controls of beneficial bacteria such as Lactobacillus and Bifidobacterium and less bacterial diversity, as well as higher levels of pathogens like Streptococcus spp., Veillonella spp. and Actinomyces spp. [52]. When Covid-19 affects elderly, immunocompromised people, it can lead to pneumonia and the development of acute respiratory distress syndrome (ARDS). Many experimental and clinical data suggest that the gut microbiota may play an important role in the development of sepsis and ARDS. Deficiencies in the immune response to SARS CoV-2 may be linked to the deprivation of some bacterial species from the intestinal tract. Many people who recovered from SARS-CoV-2 infection with COVID-19 were found to have RNA SARS-CoV-2 in their feces, which suggests that the gastrointestinal virus replication may proceed independently of the lumbar spaces. Recent study has confirmed that the intestines’ involvement in COVID-19 is more extensive and long-lasting than the respiratory system’s involvement.
The gut microbiota of roughly 100 COVID 19 patients was studied during and for up to 30 days following hospitalization to discover if the intestinal microbiome is connected with the severity of the disease in COVID-19 patients and if fluctuations in the condition are rectified by virus removal. Bacteroidetes were shown to be more numerous in people with COVID-19 than in healthy controls, but Actinobacteria were found to be more prevalent in non-COVID-19 individuals. This altered composition also showed a classification based on illness gravity, associated to higher concentrations of c reactive protein, lactatedehydrogenase, aminotransferase, gamma-glutamyl transferase, etc. Fluorobacterium dentium and Lactobacillus ruminis increased in patients who had recovered from the illness, but Bifidobacterium longum and E. rectale dropped in those who hadn’t regained their health. Cytokines and inflammatory markers, as well as worsening clinical states, may be linked to hospital-acquired gut flora composition. Although COVID-19-infected people' gut microbiota appeared to be altered, non-infected individuals' microbiota did not change much [53]. This has important implications for immunological disorders other than COVID-19 infection.
Stool microbiomes of 15 Coronavirus Disease 2019 patients were shotgun metagenomic sequenced in a comparable study and found to be significantly different from those of controls, according to that study's findings. A dysbiotic gut and fewer symbionts persisted even after the SARS-CoV-2 virus was eliminated and the respiratory symptoms abated [51].
Probiotics may improve immunity to covid 19 by increasing the activity of T suppressor cells, T helper cells, and NK cells, promoting IL-10 production, and increasing the phagocytic ability of PMN cells, among other factors.
As a result, prebiotics enhance the differentiation and reproduction of lymphocytes and macrophages, as well as the reticulo-endothelial system. To name a few of the positives, increasing the intestinal epithelial barrier and competing for resources with pathogens are all advantages [54].
Several studies have shown that probiotics, prebiotics, and synbiotics can help alleviate the symptoms of covid 19. Probiotics and synbiotics are being tested in clinical trials for their anti-viral and anti-inflammatory activities in the prevention and treatment of COVID-19. Lactobacilli and Bifidobacteria strains were the most commonly utilized in the tests because COVID-19 reduces both species. Vaccine efficacy is dependent on the presence of a balanced microbiome in the stomach. There is currently a clinical trial underway to see whether or if a yeast-based probiotic, ABBC1 (1,3/1,6-glucans and inactivated Saccharomyces cerevisiae, as well as trace minerals selenium and zinc.), can improve a COVID-19 vaccine. An increase in the efficacy of the COVID-19 immunization is expected if the gut flora is altered by supplementation.
Infectious airway disorders are controlled by microorganisms that modulate the local or distal immune system [55]. As evidenced by higher levels of bacteria, inflammation, organ dysfunction, and mortality in animals lacking in microbiota compared to controls [56], several studies have shown that the gut microbiome protects the host during pneumococcal pneumonia.
It is possible that infection with Mycobacterium tuberculosis (Mtb) and life expectancy have an impact on the composition of the gut microbiota. In the early stages of Mtb lung colonization, the gut microbiota population can act as a barrier. Bacteroids, which contain a wide range of beneficial microbiota species, were decreased in TB patients compared to healthy controls, but Actinobacteria and Proteobacteria were found to be abundant in TB patients' intestines (HCs). Many more SCFA-producing bacteria have been found in TB patients than in HCs, including Faecalibacterium, Roseburia, Eubacterium, and Phascolarctobacterium [49, 57]. Patients with active TB had lower levels of amino acid and vitamin synthesis than healthy controls, according to a functional study [58]. Patients with new and recurrent TB had their gut microbiota compared. Proteobacteria and actinobacteria were greatly increased in the intestines of persons with recurring tuberculosis, but Bacteroidetes were severely reduced. Prevotella and Lachnospira levels in TB patients were significantly lower than in healthy individuals in both newly diagnosed and recurrent cases [57]. In a recent study, the immunological response to MTB infection was studied in terms of how gut microbiota interacts with the lung. The inducible C-type expression of lung macrophages was shown to be reduced in antibiotic-induced gut dysbiosis, whereas the survival of Mtb was increased. As a result, antibiotics increase the number of Tregs in the lungs, while decreasing the number of effector and memory T cells.
Future Perspectives
The past two decades have portrayed us the importance of the role of microbes in the maintenance of health and the prevention and treatment of various diseases. Advanced and deeper studies of the microbes, including fungi, viruses, protozoa etc., are necessary since their role in the maintenance of health in humans have been critical, and yet the knowledge we currently possess regarding them is very little.
Therapeutic researches and strategies in the field of biotechnology aim to work on developing the methodology for the use of microbiome for the maintenance of equilibrium. Many of the start-up companies and multinational pharmaceutical companies showed keen interest in the usage of microbiota for determining the imbalances in human health. The biotechnological field is researching new methodologies for incorporating microbiota into Live Biotherapeutics Products for the restoration of depleted microbial products in the affected individuals.
With the help of human microbiota, characterization of new biochemical functions has been made possible for the purposes of screening, profiling and also assays for the purpose of phenotyping.
It has been discovered that the potential of the gut microbiome is extended even into the field of respiratory studies as discussed with the above evidences. For the characterization of the progression of COVID- 19 in patients, novel strategies have been developed for the modulation of the gut microbiome. One such example is the KB109 which is basically a novel synthetic glycan that increases the production of fatty acids containing short chains by the modulation of microbiota in the gut.
With the help of technological evolution, it is possible for us to find novel methods to use microbiomes for preventing and treating diseases and thus, maintain homeostasis. For the prophylactic and therapeutic management of chronic respiratory diseases, it is necessary to understand the microbiota respiratory signatures and microbiota. Since not much research is taking place on the correlation between the microbiota and respiratory diseases. This can start from identifying the correlation between the microbial flora and the host immunity. The statement has been supported by enough evidences and data which we have mentioned in the study. So, the need of the say is to drive the research on respiratory diseases on this pathology which can be used to develop therapeutic strategies that can tackle the issue to a greater extent including very severe disease like Covid-19 as we have already mentioned. Discovering new domains in this area will be a breakthrough in the field of respiratory medicine along with promoting good gut health among people. Potential clinical activities of microbial flora can be elucidated by characterizing their actions on definitve variables, both quantitative and qualitative, as this holds extensive correlation in respiratory diseases and is therefore of very much significance.
Conclusion
Due to advances in sequencing technology, the gut microbiota has become the human body's most researched microbiome. However, compared to gut microbiota research, airway microbiota research is in its infancy and has to be further clarified.
Changes in the gut microbiome homeostasis are directly linked with respiratory diseases, with their property to affect even the distal organs and thereby have a significant effect on the same. With each disease following their own molecular mechanisms, the root principle lies in the ability of the microbiome to dysregulate the immunity of the lungs and further inflammatory cascades.
Thus the development of microbiota-based therapeutics is one way to maintain homeostasis for the microbiota and the host. As our understanding of the lung's relationship to the gastrointestinal microbiome deepens, we hope to find new and better ways to treat respiratory disorders by studying how nutrition, probiotics, and faecal microbiome transplantation (FMT) can affect these microbes and their imbalance.
References
Hills RD Jr, Pontefract BA, Mishcon HR et al (2022) Gut microbiome: profound implications for diet and disease. Komp Nutr Diet. https://doi.org/10.1159/000523712
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:k2179. https://doi.org/10.1136/bmj.k2179
Feng Q, Chen W-D, Wang Y-D (2018) Gut Microbiota: an integral moderator in health and disease. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00151
Budden KF, Gellatly SL, Wood DLA et al (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15:55–63. https://doi.org/10.1038/nrmicro.2016.142
Jandhyala SM, Talukdar R, Subramanyam C et al (2015) Role of the normal gut microbiota. World J Gastroenterol 21:8787–8803. https://doi.org/10.3748/wjg.v21.i29.8787
Pushpanathan P, Mathew GS, Selvarajan S et al (2019) Gut microbiota and its mysteries. Indian J Med Microbiol 37:268–277. https://doi.org/10.4103/ijmm.IJMM_19_373
Shreiner AB, Kao JY, Young VB (2015) The gut microbiome in health and in disease. Curr Opin Gastroenterol 31:69–75. https://doi.org/10.1097/MOG.0000000000000139
Anand S, Mande SS (2018) Diet, microbiota and gut-lung connection. Front Microbiol 9:2147. https://doi.org/10.3389/fmicb.2018.02147
Cresci GAM, Izzo K (2019) Gut microbiome. In: Corrigan ML, Roberts K, Steiger E (eds) Adult short bowel syndrome. Elsevier, pp 45–54. https://doi.org/10.1016/B978-0-12-814330-8.00004-4
Graf D, Di Cagno R, Fåk F et al (2015) Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 26:26164. https://doi.org/10.3402/mehd.v26.26164
Aagaard K, Ma J, Antony KM et al (2014) The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65. https://doi.org/10.1126/scitranslmed.3008599
Rackaityte E, Halkias J, Fukui EM et al (2020) Viable bacterial colonization is highly limited in the human intestine in utero. Nat Med 26:599–607. https://doi.org/10.1038/s41591-020-0761-3
van den Elsen LWJ, Garssen J, Burcelin R, Verhasselt V (2019) Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front Pediatr 7:47. https://doi.org/10.3389/fped.2019.00047
Bolte LA, Vich Vila A, Imhann F et al (2021) Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 70:1287–1298. https://doi.org/10.1136/gutjnl-2020-322670
Rodriguez-Palacios A, Harding A, Menghini P et al (2018) The artificial sweetener splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease–like ileitis. Inflamm Bowel Dis 24:1005–1020. https://doi.org/10.1093/ibd/izy060
Moreno-Indias I, Sánchez-Alcoholado L, Pérez-Martínez P et al (2016) Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct 7:1775–1787. https://doi.org/10.1039/c5fo00886g
Rinninella E, Raoul P, Cintoni M et al (2019) What is the healthy gut Microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. https://doi.org/10.3390/microorganisms7010014
van der Merwe M (2021) Gut microbiome changes induced by a diet rich in fruits and vegetables. Int J Food Sci Nutr 72:665–669. https://doi.org/10.1080/09637486.2020.1852537
Wen L, Duffy A (2017) Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr 147:1468S-1475S. https://doi.org/10.3945/jn.116.240754
Forslund K, Hildebrand F, Nielsen T et al (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528:262–266. https://doi.org/10.1038/nature15766
Pellanda P, Ghosh TS, O’Toole PW (2021) Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr Opin Biotechnol 70:48–55. https://doi.org/10.1016/j.copbio.2020.11.001
Jangi S, Gandhi R, Cox LM et al (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015. https://doi.org/10.1038/ncomms12015
Zhao Q, Elson CO (2018) Adaptive immune education by gut microbiota antigens. Immunology 154:28–37. https://doi.org/10.1111/imm.12896
Schroeder BO (2019) Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep (Oxf) 7:3–12. https://doi.org/10.1093/gastro/goy052
Kubinak JL, Petersen C, Stephens WZ et al (2015) MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17:153–163. https://doi.org/10.1016/j.chom.2014.12.009
Nakajima A, Vogelzang A, Maruya M et al (2018) IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med 215:2019–2034. https://doi.org/10.1084/jem.20180427
Inoue R, Sakaue Y, Sawai C et al (2016) A preliminary investigation on the relationship between gut microbiota and gene expressions in peripheral mononuclear cells of infants with autism spectrum disorders. Biosci Biotechnol Biochem 80:2450–2458. https://doi.org/10.1080/09168451.2016.1222267
Richards AL, Muehlbauer AL, Alazizi A et al (2019) Gut Microbiota has a widespread and modifiable effect on host gene regulation. mSystems. https://doi.org/10.1128/msystems.00323-18
Fuess LE, den Haan S, Ling F et al (2021) Immune gene expression covaries with gut microbiome composition in stickleback. MBio. https://doi.org/10.1128/mBio.00145-21
Rosenfeld CS (2017) Gut dysbiosis in animals due to environmental chemical exposures. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00396
Defois C, Ratel J, Garrait G et al (2018) Food chemicals disrupt human gut Microbiota activity and impact intestinal homeostasis as revealed by in vitro systems. Sci Rep. https://doi.org/10.1038/s41598-018-29376-9
Espírito Santo C, Caseiro C, Martins MJ et al (2021) Gut Microbiota, in the halfway between nutrition and lung function. Nutrients 13:1716. https://doi.org/10.3390/nu13051716
Chunxi L, Haiyue L, Yanxia L et al (2020) The gut microbiota and respiratory diseases: new evidence. J Immunol Res 2020:2340670. https://doi.org/10.1155/2020/2340670
Hufnagl K, Pali-Schöll I, Roth-Walter F, Jensen-Jarolim E (2020) Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol 42:75–93. https://doi.org/10.1007/s00281-019-00775-y
Barcik W, Boutin RCT, Sokolowska M, Finlay BB (2020) The role of lung and gut microbiota in the pathology of asthma. Immunity 52:241–255. https://doi.org/10.1016/j.immuni.2020.01.007
Durack J, Kimes NE, Lin DL et al (2018) Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun. https://doi.org/10.1038/s41467-018-03157-4
Zhang J, Ma J, Li Q et al (2021) Exploration of the effect of mixed probiotics on microbiota of allergic asthma mice. Cell Immunol 367:104399. https://doi.org/10.1016/j.cellimm.2021.104399
Cereta AD, Oliveira VR, Costa IP et al (2021) Early life microbial exposure and immunity training effects on asthma development and progression. Front Med (Lausanne) 8:662262. https://doi.org/10.3389/fmed.2021.662262
Li L, Fang Z, Liu Z et al (2021) Lactobacillus reuteri CCFM1072 and CCFM1040 with the role of Treg cells regulation alleviate airway inflammation through modulating gut microbiota in allergic asthma mice. J Funct Foods 76:104286. https://doi.org/10.1016/j.jff.2020.104286
Alqahtani JS, Oyelade T, Aldhahir AM et al (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE 15:e0233147. https://doi.org/10.1371/journal.pone.0233147
Ding K, Chen J, Zhan W et al (2021) Microbiome links cigarette smoke-induced chronic obstructive pulmonary disease and dietary fiber via the gut-lung axis: a narrative review. COPD 19:10–17. https://doi.org/10.1080/15412555.2021.2019208
Walter J, Maldonado-Gómez MX, Martínez I (2018) To engraft or not to engraft: an ecological framework for gut microbiome modulation with live microbes. Curr Opin Biotechnol 49:129–139. https://doi.org/10.1016/j.copbio.2017.08.008
Enaud R, Prevel R, Ciarlo E et al (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10:9. https://doi.org/10.3389/fcimb.2020.00009
Makki K, Deehan EC, Walter J, Bäckhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715. https://doi.org/10.1016/j.chom.2018.05.012
Liu X, Cheng Y, Zang D et al (2021) The role of gut microbiota in lung cancer: From carcinogenesis to immunotherapy. Front Oncol 11:720842. https://doi.org/10.3389/fonc.2021.720842
Kipanyula MJ, Seke Etet PF, Vecchio L et al (2013) Signaling pathways bridging microbial-triggered inflammation and cancer. Cell Signal 25:403–416. https://doi.org/10.1016/j.cellsig.2012.10.014
Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR (2012) Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3:448. https://doi.org/10.3389/fphys.2012.00448
Mao Q, Jiang F, Yin R et al (2018) Interplay between the lung microbiome and lung cancer. Cancer Lett 415:40–48. https://doi.org/10.1016/j.canlet.2017.11.036
Babu N, Advani J, Solanki HS et al (2018) MiRNA and proteomic dysregulation in non-small cell lung cancer in response to cigarette smoke. MicroRNA 7:38–53. https://doi.org/10.2174/2211536607666180103165343
Zuo T, Zhang F, Lui GCY et al (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159:944-955.e8. https://doi.org/10.1053/j.gastro.2020.05.048
Spagnolello O, Pinacchio C, Santinelli L et al (2021) Targeting microbiome: an alternative strategy for fighting SARS-CoV-2 infection. Chemotherapy 66:24–32. https://doi.org/10.1159/000515344
Yeoh YK, Zuo T, Lui GC-Y et al (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–706. https://doi.org/10.1136/gutjnl-2020-323020
Walton GE, Gibson GR, Hunter KA (2021) Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br J Nutr 126:219–227. https://doi.org/10.1017/S0007114520003980
Zhang D, Li S, Wang N et al (2020) The cross-talk between gut Microbiota and lungs in common lung diseases. Front Microbiol 11:301. https://doi.org/10.3389/fmicb.2020.00301
Schuijt TJ, Lankelma JM, Scicluna BP et al (2016) The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65:575–583. https://doi.org/10.1136/gutjnl-2015-309728
Luo M, Liu Y, Wu P et al (2017) Alternation of gut microbiota in patients with pulmonary tuberculosis. Front Physiol. https://doi.org/10.3389/fphys.2017.00822
Maji A, Misra R, Dhakan DB et al (2018) Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers: gut microbiome of TB patients. Environ Microbiol 20:402–419. https://doi.org/10.1111/1462-2920.14015
Funding
This manuscript received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thottarath Prasanthan, A., Damodaran, A., Kumar, N.S. et al. Deducing the Interplay Between Gut Flora and Respiratory Diseases: A New Therapeutic Strategy?. Indian J Microbiol 63, 1–17 (2023). https://doi.org/10.1007/s12088-022-01051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-022-01051-8