Abstract
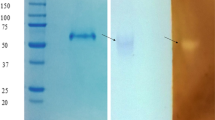
Extracellular glucoamylase of Colletotrichum sp. KCP1 produced through solid state fermentation was purified by two steps purification process comprising ammonium sulphate precipitation followed by gel permeation chromatography (GPC). The Recovery of glucoamylase after GPC was 50.40 % with 19.3-fold increase in specific activity. The molecular weight of enzyme was found to be 162.18 kDa by native-PAGE and was dimeric protein of two sub-units with molecular weight of 94.62 and 67.60 kDa as determined by SDS-PAGE. Activation energy for starch hydrolysis was 26.45 kJ mol−1 while temperature quotient (Q 10 ) was found to be 1.9. The enzyme was found to be stable over wide pH range and thermally stable at 40–50 °C up to 120 min while exhibited maximum activity at 50 °C with pH 5.0. The pKa1 and pKa2 of ionisable groups of active site controlling V max were 3.5 and 6.8, respectively. V max , K m and K cat for starch hydrolysis were found to be 58.82 U ml−1, 1.17 mg (starch) ml−1 and 449 s−1, respectively. Activation energy for irreversible inactivation (E a(d)) of glucoamylase was 74.85 kJ mol−1. Thermodynamic parameters of irreversible inactivation of glucoamylase and starch hydrolysis were also determined.







Similar content being viewed by others
References
Bhatti HN, Rashid MH, Nawaz R, Asgher M, Perveen R, Jabbar A (2007) Purification and characterization of novel glucoamylase from Fusarium solani. Food Chem 103:338–343
Pandey A (1995) Glucoamylase research: an overview. Starch 47:439–445
Norouzian D, Azim A, Jeno MS, Murrauy MY (2006) Fungal glucoamylases. Biotechnol Adv 44:80–85
Fogarty WM (1983) Microbial amylases. In: Fogarty WM (ed) Microbial enzyme and biotechnology. Applied Science Publishers, London, pp 1–90
Marlida Y, Hassan SN, Radu SZ, Baker J (2000) Purification and characterization of sago starch degrading glucoamylase from Acremonium sp. endophytic fungus. Food Chem 71:221–227
Sanroman A, Murado MA, Lema JM (1996) The influence of substrate structure on kinetics of hydrolysis of starch by glucoamylase. Appl Biochem Biotechnol 59:127–130
Gohel V, Naseby DC (2007) Thermostabilization of chitinolytic enzymes of Pantoea dispersa. Biochem Eng J 35:150–157
Prajapati VS, Trivedi UB, Patel KC (2012) Optimization of glucoamylase production by Colletotrichum sp. KCP1 using statistical methodology. Food Sci Biotechnol 22:31–38
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T-4. Nature 227:680–685
Dixon M, Webb EC (1979) Enzymes: enzyme kinetics. Academic Press, New York, pp 47–206
Eyring H, Stearn AE (1939) The application of theory of absolute reaction rates to protein. Chem Rev 24:253–270
Treichel H, Mazutti MA, Maugeri F, Rodrigues MI (2009) Technical viability of production, partial characterization of inulinase using pretreated agroindustrial residues. Bioprocess Biosys Eng 32:425–433
Muhammad R, Raheela P, Muhammad RJ, Habibullah N, Muhammad HR (2007) Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme Microb Technol 41:558–564
El-Sayed SM, El-Aassar SA, Abdel-Meguid DI (2000) Leaching, purification and some properties of glucoamylase from solid state cultures of Monascus purpureus ATCC 16437. Afr J Mycol Biotechnol 8:1–18
Koc O, Metin K (2010) Purification and characterization of thermostable glucoamylase produced by Aspergillus flavus HBF34. Afr J Biotechnol 23:3414–3424
Tosi LRO, Terenzi HF, Jorge JA (1993) Purification and characterization of an extracellular glucoamylase from thermophilic fungus Humicola grisea var. thermoidea. Can J Microbiol 39:846–852
Venkatatramu K, Manjunath P, Rao MRR (1975) Glucoamylase of Aspergillus niger NRRL 330. Ind J Biochem Biophys 12:107–114
Norouzian D, Rostami K, Nouri ID, Saleh M (2000) Subsite mapping of purified glucoamylases I, II, III produced by Arthrobotrys amerospora ATCC 34468. World J Microbiol Biotechnol 16:155–161
Mishra R, Maheshwari R (1995) Amylases of thermophilic fungus Thermomyces lanuginosus: their purification, properties, action on starch and response to heat. J Biosci 21:653–672
Zafar I, Rashid MH, Jabbar A, Malana MA, Khalid AM, Rajoka MI (2003) Kinetics of enhanced thermostability of an extracellular glucoamylase from Arachniotus sp. Biotechnol Lett 25:1667–1670
Kumar S, Satyanarayana T (2003) Purification and kinetics of raw starch hydrolyzing, thermostable and neutral glucoamylase produced by thermophilic mould Thermomucor indicae-seudaticae. Biotechnol Prog 19:936–944
Niaz M, Ghafoor MY, Jabbar A, Wahid A, Rasul E, Ahmed R (2004) Properties of glucoamylase from mesophilic fungus Arachniotus citrinus produced under solid-state growth conditions. Int J Biotechnol 1:223–231
Selvakumar P, Ashakumary L, Helen A, Pandey A (1996) Purification and characterization of glucoamylase produced by Aspergillus niger in solid state fermentation. Lett Appl Microbiol 23:403–406
James JA, Lee BH (1995) Characterization of glucoamylase from Lactobacillus amylovorus ATCC 33621. Curr Microbiol 34:186–191
Raviyan P, Tang J, Rasco BA (2003) Thermal stability of alpha-amylase from Aspergillus oryzae entrapped in polyacrylamide. J Agric Food Chem 51:5462–5466
Acknowledgments
The authors are grateful to Department of Biotechnology, Ministry of Sciences and Technology, India for providing financial assistance during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prajapati, V.S., Trivedi, U.B. & Patel, K.C. Kinetic and Thermodynamic Characterization of Glucoamylase from Colletotrichum sp. KCP1. Indian J Microbiol 54, 87–93 (2014). https://doi.org/10.1007/s12088-013-0413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-013-0413-0