Abstract
Introduction
Radiofrequency ablation (RFA) is a widely accepted, minimally invasive treatment modality for patients with hepatocellular carcinoma (HCC). Accurate prognosis prediction is important to identify patients at high risk for cancer progression/recurrence after RFA. Recently, state-of-the-art transformer models showing improved performance over existing deep learning-based models have been developed in several fields. This study was aimed at developing and validating a transformer model to predict the overall survival in HCC patients with treated by RFA.
Methods
We enrolled a total of 1778 treatment-naïve HCC patients treated by RFA as the first-line treatment. We developed a transformer-based machine learning model to predict the overall survival in the HCC patients treated by RFA and compared its predictive performance with that of a deep learning-based model. Model performance was evaluated by determining the Harrel’s c-index and validated externally by the split-sample method.
Results
The Harrel’s c-index of the transformer-based model was 0.69, indicating its better discrimination performance than that of the deep learning model (Harrel’s c-index, 0.60) in the external validation cohort. The transformer model showed a high discriminative ability for stratifying the external validation cohort into two or three different risk groups (p < 0.001 for both risk groupings). The model also enabled output of a personalized cumulative recurrence prediction curve for each patient.
Conclusions
We developed a novel transformer model for personalized prediction of the overall survival in HCC patients after RFA treatment. The current model may offer a personalized survival prediction schema for patients with HCC undergoing RFA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide, and its incidence is expected to rise further [1]. Only 20% of patients with HCC are candidates for resection [2]. Over the past two decades, image-guided minimally invasive techniques have been widely used to treat hepatocellular carcinoma, and promising results have been obtained with one such technique, namely, radiofrequency ablation (RFA), with the survival rates comparable to those of hepatectomy [3]. Over the past decade, there has been increasing interest in RFA as a useful treatment modality for patients with HCCs that were considered unresectable due to poor liver function or complications [4, 5]. Improvements in surveillance programs have reduced the number of hepatocellular carcinomas detected in advanced stages, with a corresponding increase in the use of RFA [2].
While RFA is recognized as an effective local treatment for hepatocellular carcinoma, HCC was reported to be associated with a worse prognosis as tumor size increase, due to the high incidence of recurrence [6]. Risk stratification of HCC patients undergoing RFA can help clinicians identify patients at high risk for cancer recurrence/progression after RFA. These patients may require more aggressive treatments and closer monitoring to detect and manage cancer recurrence or progression. In addition, the economic and medical burden of HCC is significant, and more effective treatment methods are needed to prolong the survival and improve the quality of life of HCC patients. Therefore, effective tools are needed for prognostic evaluation of HCC patients undergoing RFA.
Machine learning (ML) is a branch of artificial intelligence (AI) drawing on the principles of computer science and mathematics, that is focused on developing and implementing computer algorithms through the development of probabilistic or statistical models based on existing data, to maximize the predictive accuracy in many fields [7]. Recently, tremendous success has been achieved in multiple fields, from natural language processing to computer vision through the use of transformer-based models [8,9,10,11]. Transformer models show improved performance over existing deep learning-based models, and are currently accorded state-of-the-art status for natural processing [10] and computer vision tasks [9, 11]. A novel transformer model that has been developed recently for survival analysis [12] has been demonstrated to show high performance on tabular data sets as compared with deep learning-based models, such as DeepSurv [13] and DeepHit [14]. Although the transformer model appears to be a promising technology for accurate evaluation of the prognosis in HCC patients treated by RFA, there are no reports yet of prediction of the prognosis using a transformer model in HCC patients undergoing RFA.
This study was aimed at developing and validating a transformer model for predicting the overall survival in patients with HCC treated by RFA, as compared with the performance of a deep learning-based model. Such a prediction model is expected to enable prediction of the therapeutic outcomes prior to treatment and allow physicians to design personalized therapeutic strategies for individual patients.
Materials and methods
Patients
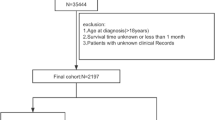
Between February 1999 and December 2019, a total of 1855 treatment-naïve HCC patients underwent RFA as the initial treatment at the Department of Gastroenterology, The University of Tokyo Hospital. Information on patient age at the start of the initial treatment, sex, serum levels of albumin, total bilirubin (TB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, alpha-fetoprotein (AFP), and Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), platelet count, prothrombin time (PT), and results of serology for hepatitis B surface (HBs) antigen and hepatitis C virus (HCV) antibody status were available for all the 1855 patients. After excluding 49 patients in whom RFA had been performed with non-curative intent to reduce the tumor burden and 28 patients for whom information on the serum levels of des-gamma-carboxyprothrombin (DCP) was lacking due to warfarin administration, we enrolled 1778 patients in the present study. The inclusion criteria for RFA were as follows: platelet count not less than 50 × 103/mm3, TB < 3 mg/dL, and prothrombin activity > 50%. Patients with macroscopic vascular invasion, refractory ascites, and/or extrahepatic metastasis were excluded from this study.
Diagnosis of HCC
The diagnosis of HCC was accomplished through the use of dynamic computed tomography (CT) or magnetic resonance imaging (MRI), with hyper-attenuation during the arterial phase and washout during the late phase considered as conclusive criteria for the diagnosis of HCC [15]. In cases where the diagnosis of HCC could not be ascertained definitively by CT, an ultrasound-guided tumor biopsy was performed, with the pathological diagnosis made on the basis of the Edmondson–Steiner criteria [16].
RFA procedure and follow-up
The RFA technique employed for the patients enrolled in this study is described elsewhere [17]. Briefly, RFA was carried out under real-time ultrasound guidance, using a single needle pass (Cool-tip®, Covidien, Boulder, CO, USA/Medtronic, Minneapolis, MN, USA, or VIVARF, STARmed®, Gyeonggi-do, Korea). Following treatment, patients were monitored for recurrence of HCC by dynamic CT or MRI at 4-month intervals; the serum AFP, DCP, and AFP-L3 levels were also recorded. Recurrence of HCC was diagnosed based on the same criteria as those applied for the diagnosis of HCC. Liver biochemistry tests were also conducted every 4 months to assess the liver function.
Model development
We applied a transformer model for survival analysis (SurvTRACE) for predicting the overall survival in patients with HCC treated by RFA [12]. The transformer model is a type of machine learning architecture that uses self-attention to process input sequences of variable lengths and capture long-term dependencies. It has been accorded state-of-the-art status for various applications, including natural language processing, precluding the need to rely on traditional sequential processing methods [8,9,10]. To develop the model, 16 potential predictor variables of the patients were employed: age; sex; daily alcohol consumption; tumor number; maximum tumor size; serum albumin (Alb), TB, AST, ALT, AFP, DCP, and AFP-L3; serology test results for HCV antibody and HBs antigen; platelet count; and prothrombin time. We standardized the data to eliminate the influences of dimensional and value range differences among the variables. For comparison, we also developed a deep learning-based model using DeepSurv. DeepSurv is a multi-layer feedforward model that draws on biological neural networks for information processing. It estimates the impact of variables on the hazard rate parameterized by the network weight, thereby serving as a predictive tool in survival analysis [13].
Statistical analysis
Continuous variables are presented as the median values with the first and third quartiles, while categorical variables are presented as numbers and frequencies (%). To evaluate the performance of the model, the 1778 patients enrolled in the study were randomly divided into three groups, as follows: (i) the training set (80%), which was used to build the model (1422 patients), (ii) the development set, which was used to determine when to stop the training process (178 patients), and (iii) the test set, which was used to evaluate the performance of each classifier (178 patients). The Harrell's c-index was determined to assess the discrimination performance of the models in both the training (internal validation) and test sets (external validation) [18]. All the prediction models were implemented and assessed in Python using SurvTRACE [12] and DeepSurv 0.2.1 [13]. The evaluative performance of each model was assessed by Kaplan–Meier curve analyses and log-rank tests across diverse risk groups using R3.6.3 (http://www.R-project.org) [19]. The significance level was set at < 0.05 for all tests.
Results
Patient characteristics
The study cohort consisted of 1778 treatment-naïve HCC patients who had undergone RFA as the primary treatment at a single institution. The patient characteristics are shown in Table 1; the median age was 70 years and approximately 60% were male. The median maximum tumor size and tumor number were 2.2 cm and 1, respectively. The patients with Child–Pugh class A accounted for 78.4% of all patients. Over a median follow-up of 50.7 months, the 5- and 10-year survival rates were calculated as 63.7% (95% confidence interval [CI] 61.4–66.1%) and 30.4% (95% CI 28.0–33.1%), respectively (Fig. 1). During the follow-up period, 1108 patients (62.3%) had died. The cause of death was HCC in 549 patients (49.5%), liver failure in 196 (17.7%), gastrointestinal bleeding in 41 (3.7%), complications related to procedure in 4 (0.4%), liver unrelated disease in 226 (20.4%), and undetermined in 92 (8.3%). The 1-, 3-, and 5-year local tumor recurrence with or without distant recurrence were 1.7%, 5.3%, and 6.5%, respectively (Supplementary Fig. 1).
Modeling process for developing the transformer model
For developing the transformer model, we applied the following hyperparameters: an epoch number of at most 100 with early stopping based on the development set performance, a batch size of 64, and a learning rate of 0.0001. The training process finished after 46 epochs, with a final validation loss of 1.16 (Supplementary Fig. 2).
Predictive performance of the transformer model in comparison with that of the deep learning model
The discriminatory performance of the predictive models was assessed by determining the Harrell’s c-index. The index values for the test set (external validation) and training set (internal validation) were 0.69 and 0.71, respectively, for the transformer model, and 0.60 and 0.59, respectively, for the deep learning-based DeepSurv model (Table 2).
Prognostic stratification of the patients using the transformer model
Figure 2 shows the Kaplan–Meier curves for survival time after the initial RFA categorized into two (Fig. 2a) and three (Fig. 2b) risk groups based on the survival estimation obtained using the transformer model. The transformer model showed a high discriminatory power for both the two and three risk categories (p < 0.001 in both risk categories).
Personalized prediction using the transformer model
Finally, we applied the transformer model for predicting the overall survival in patients with HCC treated by RFA. Supplementary Fig. 2 shows the predicted survival in two patients: a case of a 76-year-old female patient who died about 6 years after the initial RFA treatment (Supplementary Fig. 3a) and a case of a 55-year-old female patient with confirmed survival of approximately 10 years after the initial RFA treatment (Supplementary Fig. 3b). The transformer model allowed output of individual survival predictions.
Discussion
RFA plays an important role in the curative treatment of early-stage hepatocellular carcinoma because of its low invasiveness and high efficacy. The recent report from Italy showed a progressive patient aging, an incremental use of semi-annual surveillance which allowing for the detection of tiny lesions, and an increased use of radiofrequency ablation to the detriment of percutaneous ethanol injection from 2004 to 2018. Due to its minimally invasive nature and high efficacy in early-stage tumors, RFA plays an important role in the management of older patients and small hepatocellular carcinomas and may have influenced the ameliorative effect on cancer stage migration during this period[20].
Accurate prediction of the patient prognosis after RFA is a critical aspect of personalized medicine, as it is crucial for determining the most appropriate treatment plan for patients and improving the overall outcomes. For example, it can be hypothesized that patients characterized by the transformer model as having a negative prognosis are more likely to have highly malignant HCC. Such patients could potentially be optimal candidates for adjuvant or more intensive treatment. It may also aid in determining the frequency and intensity of follow-up monitoring/imaging. Several factors have been identified as prognostic indicators in HCC patients undergoing RFA, including the age, tumor size, tumor number, tumor marker, platelet count, and liver function [3, 21].
Despite the potential of the transformer model as a prognosis evaluation tool for HCC patients undergoing RFA, there are currently no reports available on its use as a prognostic prediction model in HCC patients treated by RFA; therefore, in this study, we developed a transformer-based machine learning model using potential risk factors to predict the overall survival in patients with HCC treated by RFA. To the best of our knowledge, this is the first study to report on the utility of a transformer model for predicting survival in patients with HCC. In the current study, we observed that the transformer-based model exhibited better predictive performance than DeepSurv, which had been identified previously as a robust model for predicting the survival or time-to-event [13].
The transformer model used in the current study is a neural network architecture that uses self-attention mechanisms and positional encoding to effectively model long-term dependencies in sequences of words or other tokens [8]. It has been accorded state-of-the-art status for a wide range of natural language processing tasks and is highly parallelizable, making it faster to train and able to handle larger datasets than earlier models [10]. For example, ChatGPT is a notable example of the advancement of transformer models for natural language processing and machine learning tasks [22], affirming the potential of the transformer model in various domains. The transformer model has also achieved state-of-the art status for multiple computer vision tasks [9, 11]. The ability of the transformer model to effectively model complex and long-term dependencies in data has made it a significant advancement in the field of machine learning and forecasting.
Personalization is one of the ultimate goals of modern medicine [23]. To achieve this, individualized risk prediction for each patient is critical. In the current study, we utilized the developed transformer model for predicting the prognosis in individual HCC patients undergoing RFA as the initial treatment. Notwithstanding the improved predictive performance of the transformer-based model developed in this study relative to that of the deep learning model, however, the prediction accuracy of the model is still not adequately satisfactory. The accurate prognosis prediction in HCC patients receiving RFA treatment was limited to fair to poor performance, with c-indices of 0.69 in the external validation site [24]. Lu et al. developed a prognostic nomogram for predicting the overall survival of HCC patients after RFA, and showed a c-index of 0.637 in the internal verification using the bootstrap method [25]. Accurate estimation of the survival in HCC patients undergoing RFA remains a challenge due to the significant variability of the clinical outcomes, which could also be influenced by non-hepatic comorbidities. Given that the predictive accuracy of machine learning models is influenced by the number of samples used for training [26], further research with larger sample sizes is necessary to enhance the accuracy of predictive models for clinical applications. Also, incorporating multiple heterogeneous forms of information, such as clinical data and imaging findings, through a multimodal representation model [27], could represent a valuable strategy to enhance the predictive performance.
Our study had several limitations. A primary limitation of this study was its single-center design, which restricts the generalizability of the findings—the predictive model developed in this study may not be applicable to other clinical settings. To confirm the general feasibility of the proposed transformer model, additional validation in a multi-center setting is necessary. Also, hospital volume was reported to be associated with the risk of mortality following RFA [28]. Therefore, the model can be expanded to include the hospital volume as a variable from the future multi-center study. Second, we have not publicly disseminated a web-based tool that facilitates access to the developed machine learning model. Consequently, the practical utilization of the model is currently precluded. What is significant, however, is that this is the first time that the transformer model has been demonstrated to work in the HCC field. Third, since the current study focused on RFA, biopsy was not performed for all cases, and therefore, the information regarding the differentiation of HCC is not available. Tumor location and shape of HCC were also important factors for treatment efficacy of RFA. The previous study showed an influence of tumor location on long-term outcomes following RFA. In this study, HCCs located in peri-portal-vein or peri-hepatic vein were associated with lower recurrence-free survival [29]. However, these factors were not included in the current study. Since we included the patients with multiple lesions, incorporating information on tumor localization and shape into machine learning models poses challenges due to the varying dimensions of these variables across different number of lesions. And finally, differences exist in the etiology of HCC between the current real-world setting and the study subject. The current study included patients who received radiofrequency treatment after 1999. Over the years, the prevalence of the underlying etiology of HCC has changed greatly. Although, recently, the proportion of HCC patients with non-viral etiologies has increased, reportedly accounting for about 30% of patients [30], only 20% of patients in the current study were non-viral patients.
In conclusion, we developed and validated a novel transformer model for personalized risk prediction of HCC recurrence/progression after RFA treatment. The current model has the potential to offer a personalized and precise schema for predicting the survival in HCC patients undergoing RFA treatment.
Data availability
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AFP-L3:
-
Lens culinaris agglutinin-reactive fraction of AFP
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CT:
-
Computed tomography
- DCP:
-
Des-gamma-carboxyprothrombin
- HBs:
-
Hepatitis B surface
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ML:
-
Machine learning
- MRI:
-
Magnetic resonance imaging
- PT:
-
Prothrombin time
- RFA:
-
Radiofrequency ablation
- TB:
-
Total bilirubin
References
Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol 2015;13:2140–2151
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–1236
Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569–577 (quiz 578)
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655–661
Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201–1209
Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: actual limitations and potential solutions in the future. World J Hepatol 2011;3:8–14
Sato M, Morimoto K, Kajihara S, Tateishi R, Shiina S, Koike K, et al. Machine-learning approach for the development of a novel predictive model for the diagnosis of hepatocellular carcinoma. Sci Rep 2019;9:7704
Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. Advances in neural information processing systems. 2017;30
Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, et al. An image is worth 16x16 words: transformers for image recognition at scale. arXiv preprint arXiv:201011929 2020
Devlin J, Chang M-W, Lee K, Toutanova K. Bert: pre-training of deep bidirectional transformers for language understanding. arXiv preprint arXiv:181004805 2018
Liu Z, Ning J, Cao Y, Wei Y, Zhang Z, Lin S, et al. Video swin transformer. Proc IEEE CVF Conf Comput Vis Pattern Recogn 2022;2022:3202–3211
Wang Z, Sun J. SurvTRACE: transformers for survival analysis with competing events. arXiv preprint arXiv:211000855 2021
Katzman JL, Shaham U, Cloninger A, Bates J, Jiang T, Kluger Y. DeepSurv: personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC Med Res Methodol 2018;18:1–12
Lee C, Zame W, Yoon J, Van Der Schaar M. Deephit: A deep learning approach to survival analysis with competing risks. Proceedings of the AAAI conference on artificial intelligence; 2018; 2018
Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology (Baltimore, MD) 1999;30:889–893
Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503
Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387
Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 2013;13:1–15
Garuti F, Neri A, Avanzato F, Gramenzi A, Rampoldi D, Rucci P, et al. The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int 2021;41:585–597
Lee S, Rhim H, Kim YS, Kang TW, Song KD. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int 2016;36:580–587
Aydın Ö, Karaarslan E. OpenAI ChatGPT generated literature review: Digital twin in healthcare. Available at SSRN 4308687 2022.
Blaha MJ, Blumenthal RS. Risk factors: New risk-assessment guidelines—More or less personalized? Nat Rev Cardiol 2014;11:136
Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med 2003;29:1043–1051
Lu Z, Sun Z, Liu C, Shi X, Li R, Shao W, et al. Prognostic nomogram for hepatocellular carcinoma with radiofrequency ablation: a retrospective cohort study. BMC Cancer 2021;21:751
Zacksenhouse M, Braun S, Feldman M, Sidahmed M. Toward helicopter gearbox diagnostics from a small number of examples. Mech Syst Signal Process 2000;14:523–543
Pandey G, Dukkipati A. Variational methods for conditional multimodal deep learning. 2017 International Joint Conference on Neural Networks (IJCNN); 2017. IEEE; 2017. 308–315
Lu LC, Shao YY, Kuo RN, Lin ZZ, Yeh YC, Shau WY, et al. Hospital volume of percutaneous radiofrequency ablation is closely associated with treatment outcomes for patients with hepatocellular carcinoma. Cancer 2013;119:1210–1216
Chen J, Peng K, Hu D, Shen J, Zhou Z, Xu L, et al. Tumor location influences oncologic outcomes of hepatocellular carcinoma patients undergoing radiofrequency ablation. Cancers 2018;10:378
Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol 2019;54:367–376
Funding
Open access funding provided by The University of Tokyo. This work was supported by the Health, Labour and Welfare Policy Research Grants from The Ministry of Health, Labour, and Welfare of Japan (Policy Research for Hepatitis Measures [23HC2001]).
Author information
Authors and Affiliations
Contributions
The 15 authors are justifiably credited with authorship according to the authorship criteria. In details: MS—conception, design, analysis and interpretation of data, drafting of the manuscript, final approval given; MM—data collection, final approval given; TF—data collection, final approval given; TY—data collection, final approval given; TW—data collection, final approval given; RN—data collection, final approval given; TN—data collection, final approval given; TM—data collection, final approval given; KU—data collection, final approval given; KE—data collection, final approval given; HN—data collection, final approval given; SS—data collection, final approval given; KK—supervision of research, critical revision of manuscript, final approval given; MF—supervision of research, critical revision of manuscript, final approval given; RT—data collection, critical revision of manuscript, final approval given.
Corresponding author
Ethics declarations
Guarantor of the article
Masaya Sato is the submission’s guarantor and takes responsibility for the integrity of the work as a whole, from inception to published article.
Conflict of interest
MS, MM, TF, TY, TW, RN, TN, TM, KU, KE, HN, SS, KK, MF, RT declare that they have no conflict of interest.
Ethical approval
The research design for the present study was approved by the Ethics Committee of the University of Tokyo Medical Research Center (Registration No. 2058), and the study was conducted in compliance with the ethical guidelines for epidemiological research published by The Japanese Ministry of Education, Culture, Sports, Science and Technology and The Ministry of Health, Labour, and Welfare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sato, M., Moriyama, M., Fukumoto, T. et al. Development of a transformer model for predicting the prognosis of patients with hepatocellular carcinoma after radiofrequency ablation. Hepatol Int 18, 131–137 (2024). https://doi.org/10.1007/s12072-023-10585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10585-y