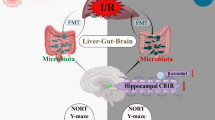
Abstract
Background
Hepatic ischemia–reperfusion injury (HIRI) is a common complication of liver surgery, which can lead to extrahepatic metabolic disorders, such as cognitive impairment. Recent observations have emphasized the critical effects of gut microbial metabolites in regulating the development of liver injury. Herein, we investigated the potential contribution of gut microbiota to HIRI-related cognitive impairment.
Methods
HIRI murine models were established by ischemia–reperfusion surgery in the morning (ZT0, 08:00) and evening (ZT12, 20:00), respectively. Antibiotic-induced pseudo-germ-free mice were gavaged with fecal bacteria of the HIRI models. Behavioral test was used to assess cognitive function. 16S rRNA gene sequencing and metabolomics were used for microbial and hippocampal analysis.
Results
Our results established that cognitive impairment caused by HIRI underwent diurnal oscillations; HIRI mice performed poorly on the Y-maze test and the novel object preference test when surgery occurred in the evening compared with the morning. In addition, fecal microbiota transplantation (FMT) from the ZT12-HIRI was demonstrated to induce cognitive impairment behavior. The specific composition and metabolites of gut microbiota were analyzed between the ZT0-HIRI and ZT12-HIRI, and bioinformatic analysis showed that the differential fecal metabolites were significantly enriched in lipid metabolism pathways. After FMT, the hippocampal lipid metabolome between the P-ZT0-HIRI and P-ZT12-HIRI groups was analyzed to reveal a series of lipid molecules with significant differences.
Conclusions
Our findings indicate that gut microbiota are involved in circadian differences of HIRI-related cognitive impairment by affecting hippocampal lipid metabolism.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic ischemia–reperfusion injury (HIRI) is one of the most common complications in the perioperative period, especially in liver surgery and transplantation [1]. In addition to influencing the hospitalization period and perioperative mortality, HIRI may also impair cognitive function in some patients, causing problems to their long-term quality of life [2]. Previous animal model studies have shown that liver injury may interfere with ammonia metabolism, causing hyperammonemia and hepatic encephalopathy, which affect cognitive function by inducing central inflammation and affecting central metabolism [3]. However, the specific mechanism of cognitive impairment caused by HIRI remains unclear.
In recent years, more and more attention has been paid to the role of gut microbiome in cognitive dysfunction. Gut microbiota plays an important role in the pathophysiological processes of various cognitive impairments related to obesity, diabetes, the postoperative phase, post-stroke, and Alzheimer’s disease [4]. Gut microbiota can influence host cognitive function impairment by regulating blood–brain barrier permeability, brain energy homeostasis, and synaptic transmission through the brain–gut axis [5]. Numerous studies have revealed associations between the gut microbiome and various types of acute liver injury (ALI) [6], induced by drugs, d-galactosamine, alcohol, and viral hepatitis. In addition, antibiotic pretreatment has been shown to alleviate HIRI and orthotopic liver transplantation outcomes in mice and humans [7], and the gut microbial metabolite to participate in the pathophysiological process of HIRI [8].
Cognitive impairment is strongly associated with circadian rhythms in both humans and animal models [9]. Liver activities, such as digestion and absorption, synthesis and uptake, assimilation, and detoxification, show significant diurnal changes, which enable their alignment with energy metabolism [10]. It is also well documented that gut microbiota undergoes diurnal oscillations both at the compositional and functional levels [11]. But while gut microbial metabolite, 1-phenyl-1,2-propanedione is involved in regulating the diurnal variation of hepatotoxicity induced by acetaminophen [12], the gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid is involved in regulating the diurnal variation of HIRI [8]. However, whether the gut microbiome is implicated in cognitive impairment associated with HIRI remains unknown.
In the present study, we explored the mechanism of the diurnal variation of HIRI-related cognitive impairment in mice, by focusing on the gut microbiota.
Materials and methods
Animals
Male-specific pathogen-free (SPF) C57BL/6 mice, aged 6–8 weeks, were housed in a temperature-controlled colony room under the standard 12 h light/dark conditions (8:00, light on; 20:00, light off), with free access to food and water ad libitum. Hepatic ischemia–reperfusion injury surgery was performed at ZT0 (8:00) or ZT12 (20:00). All mice were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd., (Beijing, China), and all experimental procedures commenced following the approval of the Institutional Animal Care and Use Committee in Tongji Hospital, Huazhong University of Science and Technology.
The mouse model of hepatic ischemia–reperfusion injury
Hepatic ischemia–reperfusion injury surgery was performed at ZT0 or ZT12 (ZT0-HIRI, n = 6; ZT12-HIRI, n = 6; ZT0-Ctr, n = 6; ZT12-Ctr, n = 6). After anesthesia with 1% pentobarbital sodium (50 mg/kg, intraperitoneal), mice were placed on an operating table, the abdominal skin was disinfected, and a median incision made to expose the liver area. The hepatic ischemia–reperfusion surgery was performed according to the method previously described [8]. Briefly, ischemia was induced by occluding the left hepatic artery and portal vein for 90 min using an artery clamp. No vascular occlusion is in sham-controlled mice. During the operation, the mice were kept warm, and the surgical incision is treated with analgesia after the operation. Behavioral tests were performed 72 h after reperfusion, and feces, liver tissue, serum, and hippocampal tissue were collected for subsequent experiments and detection.
Behavioral test
For the HIRI and control groups, behavioral experiments were performed 72 h after reperfusion. For the pseudo-germ-free mice group, behavioral experiments were performed during the next day after completion of the microbiota transfer. The open-field test, the Y-maze test, and the novel object preference test were performed to assess cognitive function. The test apparatus was wiped with 75% ethanol to eliminate odor after each experiment.
Open field test
As previously described [13], the mice were placed into the center of a black open-field chamber (L × W × H: 40 cm × 40 cm × 40 cm) after habituation. They moved freely under dim light (300 lx) for 5 min, and the total distance traveled and the time spent in the center area were analyzed.
Novel object preference test
Two identical objects were placed at two corners 6 cm from each border as previously described [13]. In the training stage, the animal was allowed to explore freely for 5 min after accommodation without objects, and the total exploration time around each object was recorded. After 2 h, the mice were placed again in the same apparatus and allowed to freely explore for 5 min with one of the identical objects being replaced with a novel object (test session). The exploration time around the novel (NT) and familiar objects (FT) was recorded and used to calculate the recognition index.
Y-maze test
As previously described [13], the Y-maze was performed with two stages in a Y-shaped compartment with three identical arms, each at an angle of 120° (L × W × H: 30 cm × 8 cm × 15 cm). The start arm (animal entry) and the common arm were always kept open, and the new arm was blocked in the first stage. Then, the mice explored the two open arms for 5 min. After 2 h, all the arms were opened, and the mice had free access to the three arms for 5 min (test trial). We recorded the time spent within each arm, the number of mice entering the new arm, and the total distance traveled by the mice for analysis.
Hematoxylin and eosin (HE) staining
Liver specimens were processed using standard HE procedures, including steps, such as dehydration, embedding, sectioning, and tissue staining. The staining was scanned on each slide and the degree of liver damage was graded using the Suzuki’s score [8].
Pseudo-germ-free mice and fecal microbial transplantation
Microbiota depletion and fecal microbiota transplantation (FMT) were performed as described previously [8]. For pseudo-germ-free mice, C57BL/6 mice received vancomycin (100 mg/kg), neomycin sulfate (200 mg/kg), metronidazole (200 mg/kg), and ampicillin (200 mg/kg) intra-gastrically once daily for 4 days. For fecal microbial transplantation, donor mice feces from the ZT0-Ctr, ZT0-HIRI, ZT12-Ctr, and ZT12-HIRI groups were collected and re-suspended in phosphate-buffered saline (PBS) to a concentration of 0.125 g/mL. This suspension was administered to mice via oral gavage (0.15 mL) daily for 3 days. Pseudo-germ-free mice receiving FMT were divided into four groups: P-ZT0-Ctr (n = 6), P-ZT0-HIRI (n = 6), P-ZT12-Ctr (n = 6), and P-ZT12-HIRI (n = 6), and the behavioral experiments were performed during the next day after FMT.
16S rRNA microbiome sequencing
After the behavioral tests, fecal samples were obtained, snap-frozen, and stored at − 80 °C. The 16S rRNA sequencing of the microbiota was performed at the OE biotech Co., Ltd. (Shanghai, China). Fecal samples were subjected to DNA extraction, amplification, library construction, and sequencing (Illumina Inc., San Diego, CA; OE Biotech Company; Shanghai, China) to obtain raw data in FASTQ format. The software and platform (https://cloud.oebiotech.cn/task/) provided by the company were used for further bioinformatic analysis of the raw data.
LC–MS analysis of fecal metabolites and lipid metabolomics in the hippocampus
The key steps of LC–MS analysis included fecal samples or hippocampus pretreatment, metabolite extraction, full-scan LC–MS detection, data pretreatment, and statistical analysis. The analytical instrument for this experiment was a Dionex U3000 UHPLC LC–MS system consisting of a QE plus high-resolution mass spectrometer, and the original LC–MS data were processed by software Progenesis QI V2.3 (Nonlinear, Dynamics, Newcastle, the UK). Variable Importance of Projection (VIP) values obtained from the OPLS-DA model were used to rank the overall contribution of each variable to group discrimination. A two-tailed Student’s T test was further used to verify whether the differences in metabolites between groups were significant. Differential metabolites were selected with VIP values greater than 1.0 and p values less than 0.05.
Statistical analysis
All the quantification data were expressed as means ± SEM, with error bars representing SEM. The normality distribution assessment was evaluated by the Kolmogorov–Smirnov test, and differences among the groups were assessed by one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test, and p < 0.05 was considered statistically significant. Statistical analyses and graphs (except for some data from 16S rRNA microbiome sequencing analyses and LC–MS) were performed with the GraphPad Prism 6.0 and Adobe Photoshop 22.1.1.
Results
Cognitive impairment caused by HIRI underwent diurnal oscillations
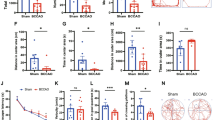
Compared with the control mice, the HIRI model showed significant differences in liver pathology (Fig. 1B), but not in serum ALT/AST and ammonia concentration (Fig. 1C). There was no diurnal difference in the liver pathological score, serum ALT/AST, and ammonia concentration (Fig. 1B, C). Compared with the ZT0, the HIRI model mice in the ZT12 group performed poorly on the Y-maze test and the novel object preference test, but not the open-field test (Fig. 1D). HIRI models also performed poorly on the Y-maze test and the novel object preference test compared with the control group when established at ZT12 (Fig. 1D). According to these findings, HIRI surgery caused severe liver injury, and induced more significant learning and short-term memory problems when surgery was performed at ZT12.
Liver injury and cognitive impairment in the mouse model of hepatic ischemia–reperfusion injury under ZT0/ZT12. Four groups: ZT0-HIRI, n = 6; ZT12-HIRI, n = 6; ZT0-Ctr, n = 6; ZT12-Ctr, n = 6. A Schematic illustration of the experimental design. B The light microscopy and Suzuki’s score of liver tissues (n = 3). Hematoxylin and eosin (H&E). Magnification × 100. C Serum levels of ALT and AST (n = 3–4); Blood ammonia concentration (n = 4). D Cognitive behavior test (open-field test, Y-maze test and novel object preference test; n = 6). One-Way ANOVA (Tukey’s multiple comparisons test); *p < 0.05, ***p < 0.001
ZT12-HIRI mice showed abnormal gut microbiota composition
16S rRNA gene sequencing technology was used to detect the composition of gut microbiota in HIRI mice. For Alpha diversity, the Simpson index did not differ between the four groups (Fig. 2A). For Beta diversity, the principal component analysis (PCA) depicted significant compositional discriminations inside the gut microbiota between the HIRI and control or ZT0 and ZT12, which revealed that gut microbial composition underwent diurnal differences and HIRI altered the microbiota composition (Fig. 2B). In addition, the microbiome composition was considerably altered after HIRI and showed diurnal differences (Fig. 2C, D). Statistical analysis was performed at the genus level, and Heatmap was drawn according to the relative abundance of different species (Fig. 2E).
The composition of gut microbiota was different between the ZT0 and ZT12 groups. A Alpha Diversity (Simpson index). B Beta Diversity (PCoA). C The relative abundance of bacteria at the genus level. All genera with an average relative abundance below 1% were grouped to “others.” D LEfSe analysis. E Heatmap of differential species at the genus level
Diurnal differences in metabolites of gut microbiota in HIRI mice
Liquid chromatography–mass spectrometry (LC–MS) was used to analyze the differences in fecal metabolites between the ZT0-HIRI and ZT12-HIRI groups. There were significant differences in OPLS-DA scores between the two groups (Fig. 3A). Compared with the ZT0-HIRI, a total of 387 metabolites of intestinal flora with significant differences were detected in the ZT12-HIRI group, including 183 which were up-regulated and 204 down-regulated (Fig. 3B). We ranked the differential metabolites based on the Variable Important in the Projection (VIP) and performed a cluster analysis of the top 50 (Fig. 3C). The top 5 down-regulated metabolites of the ZT12-HIRI group according to the VIP were Taurocholic acid 3-sulfate, L-Alloisoleucine, LysoPE(P-16:0/0:0), trans-Cinnamic acid, and 3-(3-Hydroxyphenyl)propanoic acid, while the top 5 up-regulated metabolites were 12-Ketodeoxycholic acid, Kurilensoside F, 6-Hydroxypentadecanedioic acid, (22E)-3beta-Hydroxychola-5,16,22-trien-24-oic Acid, and Diethylene glycol dimethacrylate, respectively (Fig. 3D). In addition, the KEGG pathway enrichment analysis was performed on differential metabolites which were found to be significantly enriched in lipid metabolism pathways, such as the glycero-phospholipid metabolism, sphingolipid metabolism, alpha-linolenic acid metabolism, and fat digestion and absorption (Fig. 3E).
Diurnal differences in fecal metabolites in HIRI mice. A OPLS-DA plot. B Volcano plot of differential metabolites. C Cluster plot of the top 50 differential metabolites. D Box plot of the top different metabolites. E KEGG pathway enrichment analysis of differential metabolites. T test; *p < 0.05, ***p < 0.001
Fecal microbiota transplantation (FMT) induced cognitive impairment behaviors and reshaped the gut microbiome in pseudo germ-free mice
To investigate whether diurnal differences in HIRI-related cognitive function are linked to gut microbiota, we established pseudo-germ-free mice and transplanted in them fecal microbiota from HIRI mice. There was no significant difference in the liver pathological score and serum ALT/AST between the four groups after FMT, but the mice receiving ZT12-HIRI FMT showed cognitive impairment compared with the P-ZT0-HIRI and P-ZT12-Ctr groups (Fig. 4A–C). For Alpha diversity, the Simpson index did not differ between the P-ZT12-HIRI and P-ZT12-Ctr or P-ZT12-HIRI and P-ZT0-HIRI (Fig. 4D). For Beta diversity, the principal component analysis (PCA) depicted significant compositional discriminations inside the gut microbiota between the P-ZT12-HIRI and P-ZT12-Ctr or P-ZT12-HIRI and P-ZT0-HIRI (Fig. 4E). Twenty two differentially expressed species were found, such as Lactobacillus, Anaerostipes, Escherichia–Shigella, Haemophilus, and Sphingomonas (Fig. 4F).
Microbiota from ZT12-HIRI mice induced cognitive impairment behavior and abnormal gut microbiota composition compared with ZT0-HIRI mice. Four groups: P-ZT0-HIRI, n = 6; P-ZT12-HIRI, n = 6; P-ZT0-Ctr, n = 6; P-ZT12-Ctr, n = 6. A The light microscopy and Suzuki’s score of liver tissues (n = 3). Hematoxylin and eosin (H&E). Magnification × 100. B Serum levels of ALT and AST (n = 3–4). C Cognitive behavior test (open-field test, Y-maze test and novel object preference test; n = 6). D Alpha Diversity (Simpson index). E Beta Diversity (PCoA). (F) Heatmap of differential species at the genus level. One-Way ANOVA (Tukey’s multiple comparisons test); *p < 0.05, **p < 0.01
Differences in lipid metabolomic profiles in the hippocampus of pseudo germ-free mice receiving FMT
Lipid metabolism is closely related to cognitive function, and gut microbiota can affect lipid metabolism through a variety of ways [14, 15]. In addition, the hippocampus is involved in cognitive modulation, anatomically. Meanwhile, we found that there was no significant difference in hippocampal levels of inflammatory cytokines (IL-1β, TNF-α, and NF-κB) between different groups (Supplementary Fig. 1). Given this, LC–MS was used to analyze the differences in hippocampal lipid metabolism between the P-ZT0-HIRI and P-ZT12-HIRI groups. There were significant differences in OPLS-DA scores between the two groups (Fig. 5A). Compared with the P-ZT0-HIRI, a total of 78 lipid molecules with significant differences were detected in the ZT12-HIRI, including 34 which were up-regulated and 44 down-regulated (Fig. 5B). We ranked the differential metabolites based on the VIP and performed a cluster analysis of the top 50 (Fig. 5C) and a correlation analysis of the top 50 (Fig. 5D). The top 5 up-regulated metabolites of the P-ZT12-HIRI group according to the VIP were PC(34:1), PI(16:0/18:1), PC(36:4), PC(38:4), and PE(16:0p/22:6), while the top 5 down-regulated metabolites were So(d18:0), So(d20:0), PC(32:0), PC(16:0/16:0) + CH3COO, and PI(18:0/20:4), respectively (Fig. 5E). In addition, the KEGG pathway enrichment analysis was performed on differential metabolites, which were found to be significantly enriched in lipid metabolism pathways, such as glycero-phospholipid metabolism, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes (Fig. 5F).
Lipidomics perturbations in the hippocampus of mice transplanted with fecal microbiota of the HIRI model. A PCA (principal component analysis). B Volcano plot of differential metabolites. C Cluster plot of the top 50 differential metabolites. D Pearson correlation coefficient of the top 20 differential metabolites. Red/Blue indicates a positive/negative correlation, respectively, and blue indicates a correlation. The size of the dots represents the magnitude of the correlation coefficient. E Box plot of the top different metabolites. F KEGG pathway enrichment analysis of differential metabolites. PE Phosphatidylethanolamine, PC Phosphatidylcholine, PI Phosphatidylinositol, LPE Lysophosphatidylethanolamine; PMe Phosphatidylmethanol, PS Phosphatidylserine, LPC Lysophosphatidylcholine, TG Triglyceride. T test; *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Liver is a major metabolic organ as well as the main peripheral circadian organ, and its damage may lead to dyshomeostasis of extrahepatic organs, including the central nervous system and gut [16, 17]. Our current study showed that HIRI caused significant liver pathological damage and induced cognitive impairment, which is consistent with earlier studies [18]. Interestingly, we illustrated that cognitive impairment caused by HIRI underwent diurnal oscillations. Specifically, the composition of gut microbiota had significant difference between the HIRI and control groups as well as diurnal differences between the ZT0 and ZT12 groups. Furthermore, gut microbiota transplantation from the ZT12-HIRI was shown to transmit cognitive impairment behavior. These findings suggest a direct association between gut microbiota and the diurnal variation of HIRI-related cognitive dysfunction.
In recent years, the circadian rhythm has attracted tremendous attention due to its substantial role in regulating physiology and behavior. Evidence from animal experiments shows that HIRI is more severe in the evening when compared with the morning after 90 min of ischemia and 6 h of reperfusion [8]. Nevertheless, clinical data from Ren et al. suggest that circadian rhythms have a significant impact on surgical outcomes of liver transplantation for patients [19]. Our current data, however, indicate that the histopathologic changes and serum ALT/AST had no significant difference in the evening (ZT12-HIRI) when compared with the morning (ZT0-HIRI) after 90 min of ischemia and 72 h of reperfusion. What did differ was HIRI-related cognitive functions. Compared with the ZT0-HIRI, the ZT12-HIRI group performed poorly on the Y-maze test and the novel object preference test due to impaired spatial working and reference memory and non-spatial visual learning memory. The observed change in histopathology and serum transaminase levels between the ZT0 and ZT12 in this study is not consistent with the study conducted by Chen et al. [8]. We speculate that this might be due to different reperfusion times.
Gut microbiota, in both mice and humans, exhibit diurnal oscillations that are influenced by feeding rhythms, and involved in the regulation of a variety of pathophysiological activities [20, 21]. The compositions of gut microbiota and metabolites, such as SCFAs, secondary bile acids (Bas), and 5-hydroxytryptamine (5-HT), are closely related to systemic metabolism and function, and related studies have shown that gut microbiota have potential neuroprotective effects [22]. We showed that the composition of gut microbiota had diurnal differences between the ZT0-HIRI and ZT12-HIRI. The limitation is that our current results can only suggest that alterations in the gut microbiota may be the result of a combination of hepatic ischemia–reperfusion and circadian rhythms. To further explore the relationship between gut microbiota and diurnal differences in HIRI-related cognitive function, we established pseudo-germ-free mice and transplanted in them fecal microbiota from HIRI mice. We found that gut microbiota transplantation from the ZT12-HIRI influenced cognitive impairment behavior. Microbial metabolites are one of the important mechanisms for gut-to-brain communication [23]. We analyzed the differences in metabolites of gut microbiota between the ZT0-HIRI and ZT12-HIRI, and a total of 387 metabolites of intestinal flora with significant differences were detected in the ZT12-HIRI compared with the ZT0-HIRI, including 183 which were up-regulated and 204 down-regulated. Among them, some differentially expressed metabolites, such as LysoPE, PC, and LysoPC, had been shown to be involved in the regulation of cognitive impairment [24]. The KEGG pathway enrichment analysis demonstrated that the differential metabolites were significantly enriched in lipid metabolism pathways, such as the glycero-phospholipid metabolism, sphingolipid metabolism, alpha-linolenic acid metabolism, and fat digestion and absorption. In addition, to investigate whether the differences in gut microbiota composition and metabolites between ZT0 and ZT12 were related to feeding, we calculated the food consumption of two groups (ZT0-HIRI vs. ZT12-HIRI) during the first 72 h of reperfusion and found that there were no significant differences in food intake among them (Data are not presented in this article). However, since we do not further compare the gut microbiota and metabolites between the two groups of pseudo-germ-free that had been kept under the same primary condition following FMT, we cannot fully determine the interference of feeding on the experimental results.
Previous studies have associated liver-related cognitive impairment with blood ammonia concentration and hippocampal inflammatory factors [3]. Our experimental data showed that there were no significant differences in blood ammonia concentration and hippocampal levels of inflammatory cytokines (IL-1β, TNF-α, and NF-κB) between ZT0-HIRI and ZT12-HIRI groups as well as P-ZT0-HIRI and P-ZT12-HIRI groups. So we speculated that there may be other mechanisms affecting circadian oscillation in HIRI-related cognitive function. Lipid metabolism is closely related to liver metabolism, gut microbiota, and cognitive function [25]. Compared to conventional mice, germ-free mice on chow-diet display reduced levels of multiple lipid molecules, concomitant with increased liver cholesterol, and decreased triglyceride content [14]. Cross-validation analysis of data from a Life-Lines-DEEP study reported that microbiota explained 4.5% of the variance in body mass index, 6% in triglycerides, and 4% in high-density lipoproteins [26]. In terms of cognitive function, a study by Bowers et al. indicated that lipid metabolism disorder in the hippocampus is a key factor affecting neural stem/progenitor cell activity and cognitive function [15]. Data from Li et al. also indicated that neuronal cholesterol metabolism in the hippocampus is closely related to memory histone acetylation-mediated memory [27]. By analyzing lipid metabolism in the hippocampus of FMT treated germ-free mice, we found a large number of differentially expressed lipid molecules between the P-ZT0-HIRI and P-ZT12-HIRI. Compared with the P-ZT0-HIRI, a total of 78 lipid molecules with significant differences were detected in the ZT12-HIRI, including 34 which were up-regulated and 44 down-regulated, suggesting that gut microbiota might affect cognitive function in HIRI mice by altering lipid metabolism in the central nervous system. As a limitation, our current study does not examine hippocampal circadian genes and signaling pathways, such as the light signal and the CLOCK genes, which have previously linked circadian oscillations, and hippocampus and cognitive functions [28,29,30].
Conclusions
To summarize, our findings show that HIRI-related cognitive impairment undergoes diurnal oscillations, and gut microbiota may be involved in circadian differences of cognitive function by affecting hippocampal lipid metabolism. Our current study provides new insights into the “liver-gut-brain” axis in the context of HIRI.
Data availability
All relevant data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Guan Y, Yao W, Yi K, Zheng C, Lv S, Tao Y, et al. Nanotheranostics for the management of hepatic ischemia-reperfusion injury. Small. 2021;17(23):e2007727
Saidi RF, Kenari SK. Liver ischemia/reperfusion injury: an overview. J Investig Surgery Off J Acad Surg Res. 2014;27(6):366–379
Jamshidzadeh A, Heidari R, Abasvali M, Zarei M, Ommati MM, Abdoli N, et al. Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother = Biomedecine & pharmacotherapie. 2017;86:514–520
Leigh SJ, Morris MJ. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta. 2020;1866(6):165767
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96
Rodriguez-Diaz C, Taminiau B, Garcia-Garcia A, Cueto A, Robles-Diaz M, Ortega-Alonso A, et al. Microbiota diversity in nonalcoholic fatty liver disease and in drug-induced liver injury. Pharmacol Res. 2022;182:106348
Nakamura K, Kageyama S, Ito T, Hirao H, Kadono K, Aziz A, et al. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J Clin Investig. 2019;129(8):3420–3434
Li R, Xie L, Li L, Chen X, Yao T, Tian Y, et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta pharmaceutica Sinica B. 2022;12(1):182–196
Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028
Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol. 2019;71(1):200–211
Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020;16(12):731–739
Gong S, Lan T, Zeng L, Luo H, Yang X, Li N, et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018;69(1):51–59
Li Z, Sun T, He Z, Li Z, Zhang W, Wang J, et al. SCFAs ameliorate chronic postsurgical pain-related cognition dysfunction via the ACSS2-HDAC2 axis in rats. Mol Neurobiol. 2022;59(10):6211–6227
Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24(12):4948–4959
Bowers M, Liang T, Gonzalez-Bohorquez D, Zocher S, Jaeger BN, Kovacs WJ, et al. FASN-dependent lipid metabolism links neurogenic stem/progenitor cell activity to learning and memory deficits. Cell Stem Cell. 2020;27(1):98-109 e111
Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, et al. A proteomics landscape of circadian clock in mouse liver. Nat Commun. 2018;9(1):1553
Ahluwalia V, Wade JB, Thacker L, Kraft KA, Sterling RK, Stravitz RT, et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol. 2013;59(3):467–473
Ma G, Chen C, Jiang H, Qiu Y, Li Y, Li X, et al. Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomed Pharmacother = Biomedecine & pharmacotherapie. 2017;90:62–68
Ren SS, Xu LL, Wang P, Li L, Hu YT, Xu MQ, et al. Circadian rhythms have effects on surgical outcomes of liver transplantation for patients with hepatocellular carcinoma: a retrospective analysis of 147 cases in a single center. Transpl Proc. 2019;51(6):1913–1919
Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529
Olson CA, Iniguez AJ, Yang GE, Fang P, Pronovost GN, Jameson KG, et al. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host Microbe. 2021;29(9):1378-1392 e1376
Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11(1):855
Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, et al. Diet and the microbiota-gut-brain axis: sowing the seeds of good mental health. Adv Nutr. 2021;12(4):1239–1285
Llano DA, Devanarayan V, Alzheimer’s Disease Neuroimaging I. Serum Phosphatidylethanolamine and Lysophosphatidylethanolamine levels differentiate Alzheimer’s disease from controls and predict progression from mild cognitive impairment. J Alzheimers Dis. 2021;80(1):311–319
Leclercq S, Le Roy T, Furgiuele S, Coste V, Bindels LB, Leyrolle Q, et al. Gut microbiota-induced changes in beta-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 2020;33(2):108238
Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817–824
Li X, Zhang J, Li D, He C, He K, Xue T, et al. Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron. 2021;109(6):957-970 e958
Sherman SM, Mumford JA, Schnyer DM. Hippocampal activity mediates the relationship between circadian activity rhythms and memory in older adults. Neuropsychologia. 2015;75:617–625
Eckel-Mahan KL. Circadian oscillations within the hippocampus support memory formation and persistence. Front Mol Neurosci. 2012;5:46
Ruby NF. Suppression of circadian timing and its impact on the hippocampus. Front Neurosci. 2021;15:642376
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873467, 81670240).
Author information
Authors and Affiliations
Contributions
ZH, HX, and JX: conceived the project and designed the experiments; ZH, YL, ZL, TS, and ZL: performed the experiments; ZH, YL, and AM: analyzed the data; ZH, HX, and JX: wrote the manuscript. All authors revised and approved the final version of the article.
Corresponding authors
Ethics declarations
Conflict of interest
Zhigang He, Yanbo Liu, Zhen Li, Tianning Sun, Zhixiao Li, Anne Manyande, Hongbing Xiang, Jun Xiong declare that they have no conflict of interest.
Ethics approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee in Tongji Hospital, Huazhong University of Science and Technology.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Z., Liu, Y., Li, Z. et al. Gut microbiota regulates circadian oscillation in hepatic ischemia–reperfusion injury-induced cognitive impairment by interfering with hippocampal lipid metabolism in mice. Hepatol Int 17, 1645–1658 (2023). https://doi.org/10.1007/s12072-023-10509-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10509-w