Abstract
Objective
To investigate the independent risk factors for the first recurrence after endovascular management in patients with Budd–Chiari syndrome (BCS), and to establish a prediction model for predicting recurrence in target patients.
Methods
BCS patients who underwent endovascular treatment in the Affiliated Hospital of Xuzhou Medical University from January 2010 to December 2015 were retrospectively examined, with their clinical, laboratory test, and imaging data collected and analyzed. Independent risk factors for recurrence were identified, and a prediction model was established and validated.
Results
A total of 450 patients met the filtering criteria, and 102 recurred during the follow-up. The median follow-up time was 87 months, ranging from 1 to 137 months. The 1-, 3-, 5- and 10-year cumulative recurrence rate was 9.11% (6.41–11.73%), 17.35% (13.77–20.78%), 20.10% (16.30–23.72%), and 23.06% (18.86–27.04%), respectively. Liver cirrhosis, ascites, thrombosis, and all the main intrahepatic drainage veins obstructed (obstructed HV + AHV) are independent risk factors, while age is an independent protective factor. The prediction model was named MRBET. Based on the model, the risk score of each patient equals (−0.385981 * Age/10) + (0.0404184 * PT) + (0.0943423 * CRE/10) + (0.0157053 * LDH/10) + (0.592179 * LC) + (0.896034 * Ascites) + (0.691346 * Thrombosis) + (0.886741 * obstructed HV + AHV), and those in the high-risk group (risk score ≥ 1.57) were more likely to recur than those in the low-risk group (HR = 6.911, p < 0.001). The MRBET model is also available as a web tool at https://mrbet.shinyapps.io/dynnomapp.
Conclusion
Liver cirrhosis, ascites, thrombosis, and obstructed HV + AHV are independent risk factors for the first recurrence; age is an independent protective factor. The prediction model can effectively and conveniently predict the risk of recurrence and screen out patients at a high recurrence risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Budd–Chiari syndrome (BCS) is characterized by obstruction at any level from the hepatic veins (HV) to the inferior vena cava (IVC) outflow [1]. In Western countries, BCS is a rare disorder that principally results from thrombosis, whose etiology has been ascribed to several factors including myeloproliferative neoplasms (MPNs), antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria (PNH), antithrombin deficiency, etc. [2,3,4]. In contrast, although there are over twenty thousand reported cases in China, the aforementioned risk factors are not common [5, 6]. Therefore, in the West, anticoagulation or TIPS is effective, while angioplasty merely works in a minority of cases [3,4,5,6,7]. In the Asia–Pacific region, symptomatic BCS with membranous or segmental obstruction accounts for a relatively high proportion, and angioplasty could benefit patients to the greatest extent regardless of stent placement [8, 9].
Over the decades, with the progress and maturity of endovascular treatment against BCS, the prognosis is generally favorable except for a fraction of patients with fulminant, acute liver failure, or other significant complications [10, 11]. Considering the favorable prognosis and chronic processing of the disease in most Chinses patients, concerns have recently been focused on those who suffer from repeated recurrence after treatment [11]. Let alone poor prognosis itself is associated with untreated recurrence [12].
Nonetheless, to the best of our knowledge, few studies have been conducted on risk factors for the recurrence of BCS. Because of the rarity of the disease, cohort studies with large sample sizes are even few and far between. This study aims to identify the independent risk factors for the first recurrence of BCS after endovascular treatment, as well as to establish and validate a prediction model and nomogram which could distinguish the risk of recurrence in patients through the analysis of 450 cases.
Patients and methods
Patients
In our study, patients with BCS who prepared for endovascular treatment were consecutively admitted to our hospital from January 2010 to December 2015. Their clinical, laboratory test, and imaging data were collected and retrospectively analyzed. The exclusion criteria were: 1. patients who have previously been diagnosed and received medical, surgical, endovascular treatment, or TIPS; 2. hepatic outflow obstruction caused by congestive heart disease, sinusoidal obstruction syndrome, or other causes; 3. significant dysfunction of vital organs such as liver, kidney, and brain; 4. secondary BCS; 5. recanalization procedure failed due to complete occlusion or complicated with old thrombi of vessel lesions; 6. patients with irregular and unstandardized anticoagulation.
Our principle of endovascular treatment is to recanalize as many veins as possible. For patients with a concurrent IVC obstruction, IVC recanalization is usually first performed. When HV recanalization was technically challenging, high risk, and could fail, the selective recanalization of the obstructed large accessory hepatic vein (AHV) (if any) could achieve an expected intrahepatic drainage effect. We applied a stepwise strategy during the procedure, with initial balloon dilation, followed by stenting when the obstructed lumen retracted > 75% or the cross-lesion pressure difference was ≥ 4 cmH2O after repeated dilation.
The primary endpoint of the study was the first recurrence after endovascular treatment. Recurrence was defined as a stenosis or occlusion in HVs, IVC or collateral veins after endovascular treatment, or relevant clinical symptoms that appear after a steady condition. All patients were followed up every 3 to 6 months from the date of diagnosis until study closure (December 31, 2020), or the death of patients, the date of the last follow-up. The state of and the duration before the first recurrence were determined by telephone follow-up and/or outpatient records. Enrolled patients were assigned to two groups: the recurrence group and the non-recurrence group.
Clinical assessment
Variables used in the analysis were selected based on the representative parameters in BCS, and relevant factors for recurrence reported previously, including gender, age, laboratory data, clinical characteristics, vascular involvement, Child–Pugh score, model of end-stage liver disease (MELD) score, and BCS-specific prognostic indices.
The criteria of diagnosis followed the BCS diagnosis and treatment specifications [1, 2]. Diagnosis was made in our center through color Doppler ultrasonography (CDUS), computed tomography (CT), magnetic resonance imaging (MRI), and/or venography. Therefore, the first available data after a definite diagnosis were used as the baseline data. Clinical characteristics, including hepatocellular carcinoma (HCC), upper gastrointestinal bleeding (UGB), liver cirrhosis (LC), and ascites, were examined by radiology or endoscopy, while hepatic encephalopathy (HE) was evaluated by the West Haven scale. The vascular involvement was evaluated by (1) whether the main intrahepatic drainage veins are obstructed, (2) whether the IVC is obstructed, and (3) whether the involved veins are complicated with thrombosis. The main intrahepatic drainage veins obstruction was further subclassified as (1) all the main intrahepatic drainage veins were obstructed; (2) at least one main intrahepatic drainage vein was patent. The main intrahepatic drainage veins include three main hepatic veins (left, middle, and right HV) and large patent AHVs (if any). AHV is defined as a HV with a diameter ≥ 5 mm in the third portal hilum [13, 14]. Child–Pugh score, MELD score, and BCS-specific scores (Clichy PI and Rotterdam BCS index) were calculated as reported [7, 15,16,17].
Statistical analysis
The modeling process is summarized in Supplementary Fig. 1. The primary endpoint of interest is the first recurrence time after endovascular treatment. Multiple strategies were applied to ensure reliable estimation of the variable effect in fitting the global model, and variables (1) presenting strong collinearity (|r|≥ 0.5); (2) with low occurrence (HE & HCC); (3) derived from individual variables (Child–Pugh score, MELD score, Clichy PI, and Rotterdam BCS index); (4) with a p value > 0.2 in the univariate screening were excluded.
The global multivariate Cox regression model was fitted with all variables that passed filtering. Age, ALT, total bilirubin (TBIL), creatinine (CRE), albumin (ALB), lactate dehydrogenase (LDH), and gamma-glutamyl transpeptidase (GGT), were scaled down by a factor of 10 for better interpretation of the estimated effect. The reduced model was constructed through backward eliminations (BE) of the global model, with the Akaike information criterion (AIC) as the stopping rule. The modeling stability was evaluated with 1000 times bootstrap. The C-statistics and calibration curve were, respectively, applied to measure the discriminative and calibrating competence of the model. C-statistics was adjusted with rms::validate() to alleviate optimism. The risk score of each patient is calculated by a formula constructed from the variables in the reduced model. The coefficient of each variables was extracted using the stats::coef() function. The risk score calculator based on the formula is provided in Supplementary Table 1 for easy application. The survminer::surv_cutpoint() function was used to determine the optimal cutoff of the risk score for risk stratification. The model was presented as both a regression formula and a nomogram. The web tool was built and deployed to shinyapp.io using the DynNom package. All statistical analyses were performed with R software (version 4.0.3), and the significant level (α) was set at 0.05 for all statistical tests for significance.
Results
Patient characteristics and follow-up results
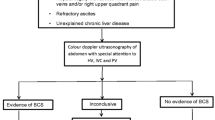
From January 2010 to December 2015, 617 BCS patients were admitted to our center and planned for endovascular treatment. Complete medical record materials of 547 patients could be retrieved. Of these patients, 80 had previously undergone surgery, endovascular treatment, or TIPS, 3 had canceled endovascular treatment due to liver and kidney failure caused by acute BCS, and 2 had secondary BCS caused by liver metastatic tumor-induced HV compression. Also, endovascular treatment failed in six patients, including three cases with whole-range occlusion of IVC, two cases complicated with old IVC thrombus, and one case with hepatic vein atrophy. Moreover, six cases did not receive standardized anticoagulant therapy according to medical advice. Finally, a total of 97 patients were excluded resulting in 450 patients included for modeling. The flowchart of this study is shown in Fig. 1.
During the follow-up period, 21 patients were lost to follow-up, and 32 patients died before recurrence. Of the dead patients, 19 were complicated with HCC at admission or newly developed HCC after discharge, 3 died of UGB, 3 died of severe hepatic encephalopathy, 1 died of lung cancer, 1 died of esophageal cancer, and 1 died of cerebral infarction.
The median follow-up time was 87 months, ranging from 1 to 137 months. The 1-, 3-, 5-, and 10-year recurrence rate was 9.11% (6.41–11.73%), 17.35% (13.77–20.78%), 20.10% (16.30–23.72%), and 23.06% (18.86–27.04%), respectively (Fig. 2). Notably, those patients who had a recurrence within 5 years after treatment accounted for 74.51% (76/102) of all the recurrent patients. Only 7.0% of patients who were followed up for more than 5 years had a recurrence. The difference between 3-, 5-, and 10-year recurrence rates showed no statistical significance (all p > 0.05). The baseline characteristics of the recurrence and the non-recurrence group are summarized in Table 1.
Prediction model
After the univariate screening (see “Patients and methods”, Fig. 3, Table 2), the global model was fitted with age, PT, ALT, PLT, TBIL, CRE, ALB, LDH, GGT, gender, LC, UGB, ascites, thrombosis, IVC, and all the main intrahepatic drainage veins obstructed (obstructed HV + AHV). Stepwise backward elimination chose the optimal model with reduced variables. The model development progress is summarized in Supplementary Fig. 1 and Table 2. In the reduced model, LC, ascites, thrombosis, and obstructed HV + AHV are independent risk factors while age is an independent protective factor. The effect of CRE (p = 0.105), PT (p = 0.099), and LDH (p = 0.119) is not significant. After internal validation using 1000-time bootstrap, the optimism-corrected C-index is 0.772, suggesting that the model has a good discriminating ability. Also, the calibration curve at 1-, 3-, and 5-year showed good calibration (Fig. 4). To quantify the patients’ risk of recurrence, we generated a formula to calculate the risk score of each patient (see “Patients and methods”): risk score = (−0.385981 * Age/10) + (0.0404184 * PT) + (0.0943423 * CRE/10) + (0.0157053 * LDH/10) + (0.592179 * LC) + (0.896034 * Ascites) + (0.691346 * Thrombosis) + (0.886741 * obstructed HV + AHV), higher value suggests higher recurrence risk. LC, ascites, thrombosis of involved veins, and obstructed HV + AHV were all binary variables, with the value of 1 (present) and 0 (absent), respectively. The risk score could be calculated automatically using the Supplementary Table 1.
For better clinical application, a nomogram was provided to estimate non-recurrence probability (Fig. 5). In the nomogram, the corresponding score can be found for each variable, and the total score of patients can be summed up. Non-recurrence probability at different time points after endovascular treatment can be speculated with the corresponding probability of the total score. We named our model as MRBET, which is short for ‘Model for Recurrence of BCS after Endovascular Treatment’. The MRBET model was also presented as an easy-to-use web tool that is freely available at https://mrbet.shinyapps.io/dynnomapp. By providing all required predictors, the recurrence probability of future patients could be predicted at any given time point.
Since this is the first study that focused on developing a prognostic model to predict the first recurrence of BCS patients after endovascular treatment, we compared this model with Child–Pugh score, MELD score, Clichy PI, and Rotterdam BCS index to justify the necessity of establishing a dedicated model. Time-ROC curves proved that the recurrence model developed in this study outperformed other non-dedicated models in predicting 3-year recurrence (Fig. 6). The area under curve (AUC) for predicting 3-year recurrence was 0.82, which was better than Child–Pugh score (0.70), Clichy PI (0.55), MELD score (0.67), and Rotterdam BCS index (0.73).
Recurrence risk stratification
The risk score was calculated for each patient who accepted endovascular treatment based on the previously obtained formula and ranged from −1.25 to 4.41. The patients with risk score value < 1.57 were stratified as the low-risk group and ≥ 1.57 as the high-risk group (“Patients and methods”). The difference in recurrence risk between the two groups was statistically significant (HR = 6.911, p < 0.001) (Fig. 7). The 1-, 3-, and 5-year recurrence rate in low-risk group was 2.65% (0.93–4.35%), 7.97% (5.04–10.81%), and 10.08% (6.81–13.24%), compared with 28.83% (19.88–6.78%), 46.03% (35.9–54.56%), and 50.87% (40.54–59.41%) in high-risk group.
Discussion
China has the highest number of diagnosed BCS patients globally, with at least 1,900 pieces of literature reporting more than 20,000 cases. However, prevalent risk factors reported in the West are relatively rare in Chinese patients [5, 6, 18]. Hence, discrepancies in clinical manifestations and treatment options of BCS exist between the two regions. Despite more than half of patients in the West being complicated with HV thrombosis, membranous or segmental obstruction is the most common in the Asia–Pacific region, which provides an opportunity to restore intrahepatic venous drainage through endovascular recanalization [8, 11, 12, 19].
In recent years, the development of endovascular treatment and materials, supported by extensive evidence-based medicine, has furthered the understanding of BCS among the physician community and improved the outcome of BCS. A meta-analysis of 2,255 patients by Zhang et al. suggested that the 1- and 6-year survival rates of patients receiving endovascular treatment were 92% (89.8–94.3%) and 76.4% (72.4–80.5%), were 87.3% (83.2–91.3%) and 72.1% (67.2–77.0%) after TIPS, respectively [20]. Meanwhile, a variety of models have been established to predict patients’ prognoses [7, 17, 21,22,23]. Unfortunately, although managing recurrent patients has constituted most of the clinical workload, few studies have been conducted on BCS recurrence, especially ones with large sample size. Additionally, Han et al. confirmed that untreated recurrence was closely associated with poor prognosis [12].
In a study involving 143 BCS patients, Cui et al. found that the 1-, 3-, and 6-year initial patency rate after endovascular treatment was 91.1%, 77.4%, and 74.0%, respectively [24]. Another study involving 177 patients showed cumulative 1-, 5-, and 10-year initial patency rates of 95%, 77%, and 58%, respectively [12]. The 1-, 3-, 5- and 10-year cumulative first recurrence rate in our study was 9.11% (6.41–11.73%), 17.35% (13.77–20.78%), 20.10% (16.30–23.72%), and 23.06% (18.86––27.04%), respectively, consistent with previous studies. It is worth mentioning that the difference between the 3-year and the 5- or 10-year recurrence rate was not statistically significant (all p < 0.05). Therefore, we suggest that the first recurrence peak after treatment is mainly within the first 3 years. Patients with no recurrence for more than 3 years are less likely to have disease progression. Compared with previous studies, the 5- and 10-year recurrence rates in this study were lower. We cautiously consider the first recurrence peak period in the first 3 years may also be that, despite the large sample size of our study, the number of cases with long-term recurrence was still limited, resulting in a wide confidence interval (95%CI) and no statistically significant difference was observed.
In the final multifactor model, liver cirrhosis, ascites, thrombosis, and obstructed HV + AHV are independent risk factors, while age is an independent protective factor (all p < 0.001). All factors included in the model could be easily obtained at the time of diagnosis, considering the feedback from the actual clinical application of some previous specific prognostic models. For instance, both Clichy PI and New Clichy PI include the clinical effect of ascites to treatment, thus impeding its use at the first diagnosis.
Patients under 30 were at higher risk of recurrence according to a study involving 471 cases between 2008 and 2012 [25]. Wang et al. demonstrated that patients aged 5 to 29 with HV involvement had the highest recurrence rate [26]. A large-scale retrospective cohort study by Li et al. also confirmed that age was a significant risk factor for recurrence after endovascular treatment in patients with IVC involvement [27]. Meanwhile, in Clichy/New Clichy PI, Rotterdam BCS index, and BCS-TIPS score, age is also included as a component [7, 17, 21, 22]. The observation above was also confirmed in our study. Nonetheless, the underlying mechanism of how age plays a protective role as an independent factor needs to be discovered.
We concluded that liver cirrhosis is an independent risk factor, consistent with a single-center study involving 130 BCS patients in China [28]. We speculate that the influence of liver cirrhosis on patients’ recurrence may be related to the following reasons: (1) hemodynamic changes: cirrhosis is characterized by diffuse proliferation of fibrous tissue. Relative stasis of blood flow in portal and hepatic venous system leads to thrombosis [29]. (2) Vascular endothelial damage: hemorrhagic cirrhosis caused by BCS results in severe congestion of internal organs, increased shear stress in the vascular wall, and disruption of the mucosal barrier of the digestive tract. Consequently, bacteria and toxins entering the circulation damage the vascular endothelium, which exposes subcutaneous tissue and activates the coagulation pathway, accelerating thrombosis in vessels or stents [30]. (3). Blood hypercoagulable state: recent studies have shown that the rebalancing blood coagulation system in patients with cirrhosis is quite fragile and can tilt toward either state of bleeding or thrombosis. The increased production of vWF and fibrinogen, changes in fibrin structure, and a low fibrinolysis state all lead to a high risk of thrombosis. This phenomenon has no significant statistical difference between liver cirrhosis with different etiology [31, 32].
Ascites, a traditional and classic indicator, is universal in predicting disease outcomes in patients with liver disease, which has been confirmed by many studies [7, 17, 21]. In our study, it is also associated with the first recurrence of patients. The presence of ascites often implies worse liver function and more severe venous obstruction, as mentioned earlier, which contributes to the recurrence.
Thrombotic events represent the progression of patients from a thrombophilic state. Under this circumstance, multiple veins are usually involved, with more distinct clinical manifestations and serious hepatic injury, leading to BCS recurrence in 5–11% of cases [33, 34]. Extensive screening for thrombogenic factors is not recommended in China according to current guidelines. But for patients with thrombosis, detection of MPNs and its related genes such as JAK2V617F, coagulation factor V Leiden, thrombin G20210A, PNH, MTHFR gene, protein C and S, and other factors is reasonable.
Obstruction of all the main intrahepatic drainage veins is an independent risk factor for recurrence. In 1952, Elias and Petty reported the existence of lower HVs outside the second hepatic portal [35]. Afterward, HVs were divided into superior and inferior groups [36]. The superior group consists of three main branches: the left, middle, and right HV, which flowed into the IVC through the second hilum. The venous trunk of the inferior group refers to as the AHV, including the caudate lobe vein and inferior right HV, which merge into the IVC through the third hilum. Caudate lobe veins are often small and undetectable, while the inferior right HV is sometimes large, which is magnitude in liver surgery and interventional procedures [37]. When BCS occurs with main HVs partially or completely obstructed, hepatic hypertension arises. In this case, AHVs sometimes compensate for dilation and act as a bridge between the portal vein (PV) and IVC to fulfill the intrahepatic drainage [38]. Plentiful studies in the past decade have confirmed that the recanalization of obstructed AHV can effectively relieve hepatic congestion and reduce liver function injury and PV pressure [39, 40]. When the main intrahepatic drainage veins, including three main HVs and AHVs (if any), are obstructed, congestive liver injury and cirrhosis aggravate, increasing the recurrence risk of patients. Generally, under this circumstance, we would manage to treat all diseased veins we observed through angiography. When there are still one or two remaining stenotic veins with unsatisfied balloon angioplasty and difficult stent placement, the intrahepatic venous drainage could still be fulfilled because most veins have been recanalized. This situation is quite common when all the main intrahepatic drainage veins are obstructed. However, the stasis of blood flow in the untreated veins results in hemodynamic changes and, ultimately, an increased risk of thrombosis. We speculate that this is one of the causes of secondary venous thrombosis and occlusion, which differs from the primary thrombophilic state in patients due to genetic mutations. There may be potential interaction between various thrombosis causes, which needs further exploration. In brief, these findings reemphasize the importance of fully recanalizing as many veins as possible.
PT, CRE, and LDH were selected by AIC and tended to be risk factors for BCS recurrence in the multivariate modeling. Although not statistically significant in our analysis, PT and CRE have repeatedly appeared in prognostic indices for BCS [7, 17], and LDH was considered an independent risk factor for BCS recurrence in a previous retrospective study [25]. Prolongation of PT reflects poor liver function, and it suggests, in part, that the fragile rebalancing blood coagulation system in BCS patients could sometimes tilt towards a state of bleeding, as mentioned in the “Discussion” [31, 32]. Despite its limitations, baseline CRE is still the most used biomarker for estimating glomerular filtration rate and assessing kidney injury in patients with cirrhosis [41]. Increased CRE implies poor liver function and thus potentially affects patients’ recurrence. LDH is an essential enzyme in the glycolytic pathway, released due to body tissue damage. The liver injury could promote LDH activities. Moreover, experiments based on large samples demonstrated higher LDH levels in MPNs and PNH, while the etiology of BCS has been ascribed to several factors, including MPNs and PNH [42, 43]. Further studies with larger sample sizes are needed to validate their clinical value.
Our prediction model, as described in “Results”, has good discrimination and calibration in predicting the first recurrence of patients with BCS after endovascular treatment, and convenient application, promising future popularization.
This study still has some limitations: (1) as a retrospective study with a long time span, recall bias will inevitably occur; (2) Although our center attracts patients from all over the country, more than half of the patients are still confined to the provincial area; the single-center research led to an unavoidable geographical shift; (3) thrombogenic factors such as JAK2V617F and MTHFR mutation were not included mainly due to the insufficient detection in our follow-up samples; and (4) there is still a lack of external validation. At present, studies on BCS with large sample size in China are only carried out by a few centers independently; multi-center cooperation is imperative.
In conclusion, liver cirrhosis, ascites, thrombosis, and obstructed HV + AHV are independent risk factors for the first recurrence of BCS patients after endovascular treatment. The prediction model can effectively and conveniently predict the risk of recurrence and screen out patients at a high recurrence risk.
Data availability
Data available on request from the authors.
References
Janssen HL, Garcia-Pagan JC, Elias E, et al. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38(3):364–371
Coilly A, Potier P, Broué P, et al. Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol. 2020;44(4):420–425
Darwish Murad S, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167–175
Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23):2452–2466
Qi X, Han G, Guo X, et al. Review article: the aetiology of primary Budd-Chiari syndrome - differences between the West and China. Aliment Pharmacol Ther. 2016;44(11–12):1152–1167
Wang H, Sun G, Zhang P, et al. JAK2 V617F mutation and 46/1 haplotype in Chinese Budd-Chiari syndrome patients. J Gastroenterol Hepatol. 2014;29(1):208–214
Darwish Murad S, Valla DC, de Groen PC, et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39(2):500–508
Cheng DL, Xu H, et al. Interventional treatment strategy for primary Budd-Chiari syndrome with both inferior vena cava and hepatic vein involvement: patients from two centers in China. Cardiovasc Intervent Radiol. 2019;42(9):1311–1321
Zhang Q, Huang Q, Shen B, Sun J, Wang X, Liu H. Efficacy and safety of endovascular intervention for the management of primary entire-inferior vena cava occlusion. Cardiovasc Intervent Radiol. 2015;38(3):665–671
Thuluvath PJ, Alukal JJ, Zhang T. A scoring model to predict in-hospital mortality in patients with Budd-Chiari syndrome. Am J Gastroenterol. 2021;116(9):1905–1912
Shukla A, Shreshtha A, Mukund A, et al. Budd-Chiari syndrome: consensus guidance of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2021;15(3):531–567
Han G, Qi X, Zhang W, He C, Yin Z, Wang J, et al. Percutaneous recanalization for Budd-Chiari syndrome: an 11-year retrospective study on patency and survival in 177 Chinese patients from a single center. Radiology. 2013;266(2):657–667
Hanaoka J, Shimada M, Uchiyama H, et al. A simple formula to calculate the liver drainage volume of the accessory right hepatic vein using its diameter alone. Surgery. 2009;146(2):264–268
Bargalló X, Gilabert R, Nicolau C, García-Pagán JC, Bosch J, Brú C. Sonography of the caudate vein: value in diagnosing Budd-Chiari syndrome. AJR Am J Roentgenol. 2003;181(6):1641–1645
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the esophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649
Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871
Zeitoun G, Escolano S, Hadengue A, et al. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30(1):84–89
Cheng D, Xu H, Lu ZJ, et al. Clinical features and etiology of Budd-Chiari syndrome in Chinese patients: a single-center study. J Gastroenterol Hepatol. 2013;28(6):1061–1067
Ding PX, Zhang SJ, Li Z, et al. Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd-Chiari syndrome. J Gastroenterol Hepatol. 2016;31(1):222–228
Zhang F, Wang C, Li Y. The outcomes of interventional treatment for Budd-Chiari syndrome: systematic review and meta-analysis. Abdom Imaging. 2015;40(3):601–608
Langlet P, Escolano S, Valla D, et al. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J Hepatol. 2003;39(4):496–501
Garcia-Pagán JC, Heydtmann M, Raffa S, et al. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135(3):808–815
Shalimar DM, Kumar A, Kedia S, et al. Hepatic venous outflow tract obstruction: treatment outcomes and development of a new prognostic score. Aliment Pharmacol Ther. 2016;43(11):1154–1167
Cui YF, Fu YF, Li DC, et al. Percutaneous recanalization for hepatic vein-type Budd-Chiari syndrome: long-term patency and survival. Hepatol Int. 2016;10(2):363–369
Gao X, Gui E, Lu Z, et al. Risk factors of recurrence among 471 Chinese patients with Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol. 2015;39(5):620–626
Wang L, Zu MH, Gu YM, et al. Budd-Chiari syndrome in children and adolescents: therapeutic radiological intervention. Chin J Pediatrics. 2013;51(8):590–594
Li WD, Yu HY, Qian AM, et al. Risk factors for and causes and treatment of recurrence of inferior vena cava type of Budd-Chiari syndrome after stenting in China: a retrospective analysis of a large cohort. Eur Radiol. 2017;27(3):1227–1237
Li G, Huang Y, Tang S, et al. A single-center retrospective study: clinical features of different types of Budd-Chiari syndrome in Chinese patients in the Hubei area. Vascular. 2018;26(1):80–89
Nery F, Correia S, Macedo C, et al. Nonselective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: results of a prospective longitudinal study. Aliment Pharmacol Ther. 2019;49(5):582–588
Carnevale R, Raparelli V, Nocella C, et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells Implications for hypercoagulability in cirrhosis. J Hepatol. 2017;67(5):950–956
O’Leary JG, Greenberg CS, Patton HM, et al. AGA Clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1
Bos S, van den Boom B, Kamphuisen PW, et al. Haemostatic profiles are similar across all aetiologies of cirrhosis. Thromb Haemost. 2019;119(2):246–253
Mentha G, Giostra E, Majno PE, et al. Liver transplantation for Budd-Chiari SYNDROME: a European study on 248 patients from 51 centres. J Hepatol. 2006;44:520
Ulrich F, Pratschke J, Neumann U, et al. Eighteen years of liver transplantation experience in patients with advanced Budd-Chiari syndrome. Liver Transpl. 2008;14(2):144–150
Elias H, Petty D. Gross anatomy of the blood vessels and ducts within the human liver. Am J Anat. 1952;90:59–111
Williams PL, Warwick R, Dyson M, Bannister LH. Gray’s anatomy. London: Churchill Livingstone; 1995
Cai SF, Gai YH, Ma S, Liang B, Wang GC, Liu QW. Ultrasonographic visualization of accessory hepatic veins and their lesions in Budd-Chiari syndrome. Ultrasound Med Biol. 2015;41:2091–2098
Yang F, Huang PC, Yan LL, Zhang ZD, Fu YF, Xia FF. Catheter aspiration with recanalization for Budd-Chiari syndrome with inferior vena cava thrombosis. Surg Laparosc Endosc Percutan Tech. 2019;29:304–307
Mammen T, Keshava S, Eapen CE, Moses V, Babu NR, Kurien G, et al. Intrahepatic collateral recanalization in symptomatic Budd-Chiari syndrome: a single-center experience. J Vasc Interv Radiol. 2010;21:1119–1124
Lv LL, Zhu LL, Chen GH, Xu P, Xu K. Recanalization of accessory hepatic vein for hepatic vein-type Budd-Chiari syndrome. Abdom Radiol (NY). 2021;46(7):3456–3463
Piano S, Brocca A, Angeli P. Renal function in cirrhosis: a critical review of available tools. Semin Liver Dis. 2018;38(3):230–241
Füreder W, Sperr WR, Heibl S, et al. Prognostic factors and follow-up parameters in patients with paroxysmal nocturnal hemoglobinuria (PNH): experience of the Austrian PNH network. Ann Hematol. 2020;99(10):2303–2313
Wimazal F, Sperr WR, Kundi M, et al. Prognostic significance of serial determinations of lactate dehydrogenase (LDH) in the follow-up of patients with myelodysplastic syndromes. Ann Oncol. 2008;19(5):970–976
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ZW and ZW contributed equally to this study. ZW designed and wrote the manuscript. ZW, ZW, and AL analyzed the data. ZW, ZW, ZZ, JL, and ZP screened the literature and collected data. JL, JG, MZ, and HX critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors Zhongkai Wang, Ziwei Wang, Zhiyuan Zhang, Jiandong Li, Zhiyang Pan, Ang Liu, Jian Lu, Jinhe Guo, Maoheng Zu, and Hao Xu declare that there is no conflict of interest.
Ethical approval
We conducted a single-center, retrospective cohort study approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Jiangsu, China; XYFY2019-KL173-01). All procedures were performed according to the Helsinki Declaration of 1975.
Informed consent
All enrolled patients gave written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Wang, Z., Zhang, Z. et al. Establishment and validation of a prediction model for the first recurrence of Budd–Chiari syndrome after endovascular treatment: a large sample size, single-center retrospective study. Hepatol Int 17, 159–169 (2023). https://doi.org/10.1007/s12072-022-10464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10464-y