Abstract
Background and aims
With metabolic dysfunction-associated fatty liver disease (MAFLD) incidence and prevalence sharply increasing globally, there is an urgent need for non-invasive diagnostic tests to accurately screen high-risk MAFLD patients for liver inflammation and fibrosis. We aimed to develop a novel sequential algorithm based on N-terminal propeptide of type 3 collagen (PRO-C3) for disease risk stratification in patients with MAFLD.
Methods
A derivation and independent validation cohort of 327 and 142 patients with biopsy-confirmed MAFLD were studied. We compared the diagnostic performances of various non-invasive scores in different disease states, and a novel sequential algorithm was constructed by combining the best performing non-invasive scores.
Results
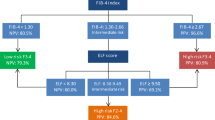
For patients with high-risk progressive steatohepatitis (i.e., steatohepatitis + NAFLD activity score ≥ 4 + F ≥ 2), the AUROC of FAST score was 0.801 (95% confidence interval (CI): 0.739–0.863), and the negative predictive value (NPV) was 0.951. For advanced fibrosis (≥ F3) and cirrhosis (F4), the AUROCs of ADAPT and Agile 4 were 0.879 (95%CI 0.825–0.933) and 0.943 (95%CI 0.892–0.994), and the NPV were 0.972 and 0.992. Sequential algorithm of ADAPT + Agile 4 combination was better than other combinations for risk stratification of patients with severe fibrosis (AUROC = 0.88), with similar results in the validation cohort. Meanwhile, in all subgroup analyses (stratifying by sex, age, diabetes, NAS, BMI and ALT), ADAPT + Agile 4 had a good diagnostic performance.
Conclusions
The new sequential algorithm reliably identifies liver inflammation and fibrosis in MAFLD, making it easier to exclude low-risk patients and recommending high-risk MAFLD patients for clinical trials and emerging pharmacotherapies.




Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AAR:
-
Aspartate aminotransferase to alanine aminotransferase ratio
- APRI:
-
Aspartate aminotransferase-platelet ratio index
- AUROC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DCA:
-
Decision curve analysis
- FIB-4:
-
Fibrosis-4
- FLIP:
-
Fatty liver inhibition of progression
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- NFS:
-
NAFLD fibrosis score
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRO-C3:
-
N-terminal propeptide of type 3 collagen
- VCTE:
-
Vibration-controlled transient elastography
References
Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022;20:e573–e582
Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hep Intl. 2020;14:889–919
Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J et al. From NAFLD to MAFLD: a "redefining" moment for fatty liver disease. Chin Med J. 2020;133:2271–2273
Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7:388–390
Méndez-Sánchez N, Díaz-Orozco L, Córdova-Gallardo J. Redefinition of fatty liver disease from NAFLD to MAFLD raised disease awareness: Mexican experience. J Hepatoly. 2021;75:221–222
Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease.Gastroenterol. 2020;158:1999-2014.e1991
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904
Zheng KI, Eslam M, George J, Zheng MH. When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr. 2020;9:801–804
Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J. 2021;134:2911–2921
Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022;18:259–268
Fouad Y, Gomaa A, Semida N, Ghany WA, Attia D. Change from NAFLD to MAFLD increases the awareness of fatty liver disease in primary care physicians and specialists. J Hepatol. 2021;74:1254–1256
Zheng KI, Zheng MH. The uprising of metabolic dysfunction-associated fatty liver disease (MAFLD) in acute-on-chronic liver failure (ACLF). Hepatobiliary Surg Nutr. 2021;10:857–859
Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatol. 2015;61:1547–1554
Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112
Zheng KI, Sun DQ, Jin Y, Zhu PW, Zheng MH. Clinical utility of the MAFLD definition. J Hepatol. 2021;74:989–991
Wu XX, Zheng KI, Boursier J, Chan WK, Yilmaz Y, Romero-Gómez M et al. acNASH index to diagnose nonalcoholic steatohepatitis: a prospective derivation and global validation study. EClinicalMedicine. 2021;41:101145
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–315
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterol. 2019;156:1264-1281.e1264
Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod pathol 1998;11:560–565
Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterol. 2005;128:1898–1906
Tang LJ, Ma HL, Eslam M, Wong GL, Zhu PW, Chen SD et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabolism: Clin Exp. 2022;128:154958
Eslam M, Wong GL, Hashem AM, Chan HL, Nielsen MJ, Leeming DJ et al. A sequential algorithm combining ADAPT and liver stiffness can stage metabolic-associated fatty liver disease in hospital-based and primary care patients. Am J Gastroenterol. 2021;116:984-993 2021;116:984–993
Younossi ZS, Newsome P, Chan W, et al. Development and validation of Agile 3+: novel FibroScan based score for the diagnosis of advanced fibrosis in patients with nonalcoholic fatty liver disease. J Hepatol. 2021;75:S205–S293.
Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209
Zhou YJ, Gao F, Liu WY, Wong GL, Mahadeva S, Raihan Nik Mustapha N et al. Screening for compensated advanced chronic liver disease using refined Baveno VI elastography cutoffs in Asian patients with nonalcoholic fatty liver disease. Alimentary Pharmacol Ther. 2021;54:470–480
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol. 2005;41:1313–1321
Fukusato T, Fukushima J, Shiga J, Takahashi Y, Nakano T, Maeyama S et al. Interobserver variation in the histopathological assessment of nonalcoholic steatohepatitis. Hepatol Res. 2005;33:122–127
Bedossa P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatol. 2014;60:565–575
Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A et al. ADAPT: An algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatol. 2019;69:1075–1086
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatol. 2003;38:518–526
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol. 2006;43:1317–1325
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatol. 2007;45:846–854
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterol. 2015;149:389–397.e310
Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatol. 2018;67:1754–1767
Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterol. 2016;150:1147–1159.e1145
Wang TY, George J, Zheng MH. Metabolic (dysfunction) associated fatty liver disease: more evidence and a bright future. Hepatobiliary Surg Nutr. 2021;10:849–852
Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol. 2019;17:630–637.e638
Noureddin M, Truong E, Gornbein JA, Saouaf R, Guindi M, Todo T et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol. 2022;76:781–787
Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterol. 2016;150:626–637.e627
Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol 2018;53:819–826
Funding
This paper was funded by grants from the National Natural Science Foundation of China (82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032) and Project of New Century 551 Talent Nurturing in Wenzhou. GT is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy. CDB is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK. Vincent Wong is supported in part by a Direct Grant from The Chinese University of Hong Kong (2020.045). ME and JG are supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206), an Investigator Grant (APP1196492) and Project and ideas grants (APP2001692, APP1107178 and APP1108422).
Author information
Authors and Affiliations
Contributions
L-JT: conceptualization, investigation, data curation, and writing—original draft. GL: conceptualization and writing—original draft. ME: investigation, writing—review and editing. P-WZ: data curation, writing—review and editing. S-DC: data curation, writing—review and editing. HH-WL: data curation and writing—review and editing. O-YH: data curation and writing—review and editing. GL-HW: investigation and writing—review and editing. Y-JZ: investigation and writing—review and editing. MK: investigation and writing—review and editing. DJL: investigation and writing—review and editing. PJ: investigation and writing—review and editing. CW: investigation and writing—review and editing. H-YY: investigation, writing—review and editing. CDB: investigation, writing—review and editing. GT: investigation and writing—review and editing. JG: conceptualization, methodology, and writing—review and editing. VW-SW: conceptualization, methodology, investigation, writing—review and editing, and project administration. M-HZ: conceptualization, methodology, investigation, data curation, writing—review and editing, and project administration. All authors contributed to the manuscript for important intellectual content and approved the final submission of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Although the PRO-C3 ELISA test was carried out at Nordic Bioscience under a research collaboration, we confirm that cohort generation, research conceptualization, analysis, and manuscript drafting were carried out independently of the Nordic Bioscience team. ADAPT has not been developed as a proprietary test. The PRO-C3 ELISA test is not currently commercially available, but can be obtained as a Nordic Bioscience research test for research use only. Diana Julie Leeming and Morten Karsdal are employed by, and own stock at Nordic Bioscience. Grace Lai-Hung Wong and Vincent Wai-Sun Wong have served as speakers and/or consultants for Echosens. Liang-Jie Tang, Gang Li, Mohammed Eslam, Pei-Wu Zhu, Sui-Dan Chen, Howard Ho-Wai Leung, Ou-Yang Huang, Yu-Jie Zhou, Pei Jiang, Cong Wang, Hai-Yang Yuan, Christopher D. Byrne, Giovanni Targher, Jacob George and Ming-Hua Zheng have no conflicts of interest.
Informed consent
The study was approved by the institutional review boards of both centres and all participants gave their written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1.
The AUROC of FAST, ADAPT and Agile 4 scores in the derivation cohort. (TIF 622 KB)
Supplementary Figure 2.
The dual cut-off approach of FAST, ADAPT and Agile 4 scores in the derivation cohort. (TIF 469 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, LJ., Li, G., Eslam, M. et al. N-terminal propeptide of type 3 collagen-based sequential algorithm can identify high-risk steatohepatitis and fibrosis in MAFLD. Hepatol Int 17, 190–201 (2023). https://doi.org/10.1007/s12072-022-10420-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10420-w