Abstract
Background
Molecular therapies and precision medicine are expected to be developed for liver cancer based on the diagnosis of DNA somatic alterations. However, it remains unclear whether TERT promoter mutation (TERT C228T) in serum cfDNA is useful for the diagnosis of liver cancer with non-viral fatty liver disease (FLD).
Methods
This retrospective cohort study examined 258 Japanese patients who had a confirmed diagnosis of primary liver cancer. We investigated the factors associated with TERT C228T and abnormal levels of liver cancer-specific tumor markers (AFP and PIVKAII) in serum samples.
Results
Multivariate analysis identified the etiology of FLD, vascular invasion, and non-cirrhosis as determinants of TERT C228T-positive liver cancer. Rates of positive TERT C228T in FLD were significantly higher than those of HBV and HCV. Conversely, rates of abnormal AFP in FLD were significantly lower than those of HBV and HCV. Viral suppression of HBV/HCV and alcohol intake did not affect TERT C228T, but AFP was significantly reduced by viral suppression. The rates of positive TERT C228T were significantly lower in HCV patients with viral clearance than those of FLD patients.
Conclusion
Our results highlight the importance of serum TERT C228T for the detection of non-viral FLD-related liver cancer. TERT C228T is a tumor marker that might not be influenced by inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of molecular therapies and precision medicine is expected for liver cancer based on the diagnosis of DNA somatic alterations. Genomic studies have identified telomerase reverse transcriptase (TERT), tumor protein p53, and catenin beta 1 as the most frequently mutated genes in liver cancer [1,2,3]. TERT promoter mutation is the most frequent genetic alteration in liver cancer [3, 4]. Furthermore, two hotspots of TERT promoter mutations, C228T and C250T, have been detected in 94.7% and 5.3% of patients with identified mutations, respectively. Thus, a stronger impact of C228T than C250T is presumed in liver cancer [5]. Recent reports show that NAFLD-related liver cancer might be less responsive to immune checkpoint inhibitors [6]. Furthermore, Wnt/CTNNB1 mutation (one type of DNA somatic alteration) might be a biomarker that could predict resistance to such therapies [7].
Our recent report highlighted the better performance of TERT C228T in serum cfDNA than AFP and PIVKAII in the early diagnosis of primary liver cancer in patients with non-alcoholic fatty live disease (NAFLD) [8]. AUROC, sensitivity, specificity, PPV, and NPV of TERT C228T were 0.812, 63.9%, 95.2%, 95.8%, and 60.6% in predicting NAFLD-related liver cancer, respectively. Those of PIVKAII positivity were 0.735, 36.1%, 66.7%, 65.0%, and 37.8%, respectively. Those of AFP positivity were 0.507, 36.1%, 66.7%, 65.0%, and 37.8%, respectively. Namely, in predicting NAFLD-related liver cancer, kappa coefficients were 0.528, 0.389, and 0.024 in TERT C228T, PIVKAII positivity, and AFP positivity, respectively [8]. However, it remains unclear whether serum TERT C228T is useful for the diagnosis of non-viral fatty liver disease (FLD)-related liver cancer, which has had an increasing trend recently [9]. Thus, the purpose of the present retrospective study was to determine the clinical and histopathological factors associated with TERT C228T in serum samples, as well as to investigate the useful marker for the diagnosis of FLD-related liver cancer.
Materials and methods
Patients
This retrospective cohort study examined 258 Japanese patients. The patients were confirmed to have a diagnosis of primary liver cancer for the first time through imaging studies between 1984 and 2020 at Toranomon Hospital. There were 117 patients who also underwent surgical resection and had a confirmed diagnosis of liver cancer with histopathological examination. Table 1 summarizes the characteristics of the 258 patients. We investigated the clinical and histopathological factors associated with TERT C228T in serum samples obtained at the first diagnosis of primary liver cancer.
The following criteria were used to select 90 patients with HBV-related liver cancer: (1) a positive test for HBV surface antigen (Chemiluminescent Enzyme Immunoassay, Abbott Laboratories, Tokyo, Japan), (2) a negative test for HCV antibody by third-generation enzyme immunoassay (Chiron Corp, CA, USA), (3) history of mild to moderate alcohol intake (estimated lifetime cumulative alcohol intake of < 500 kg), and (4) confirmed lack of hemochromatosis, Wilson disease, primary biliary cholangitis, and autoimmune liver disease. There were 57 patients who did not receive antiviral therapy (nucleos(t)ide analogues [NUCs]) and were diagnosed with liver cancer. Liver cancer was detected in the other 33 patients, regardless of the achievement of viral suppression under NUCs.
The following criteria were used to select 96 patients with HCV-related liver cancer: (1) a positive test for HCV antibody and HCV RNA by quantitative analysis before antiviral therapy (direct-acting antivirals [DAAs]), (2) negative test for HBV surface antigen, (3) history of mild to moderate alcohol intake, and (4) confirmed lack of hemochromatosis, Wilson disease, primary biliary cholangitis, and autoimmune liver disease. Sustained virological response (SVR) regarded as HCV clearance was defined as a negative HCV RNA result at 12 weeks after the cessation of DAAs according to the COBAS TaqMan HCV test (Roche Diagnostics, Tokyo, Japan). There were 30 patients who did not receive DAAs and were diagnosed with liver cancer. Liver cancer was detected in 10 patients after the diagnosis of non-SVR by DAAs, and in the other 56 patients, it was detected after the diagnosis of SVR by DAAs.
The following criteria were used to select 72 patients with FLD-related liver cancer: (1) histopathological changes of steatosis in at least 5% of hepatocytes, (2) negative test for HBV surface antigen and HCV antibody, and (3) a confirmed lack of viral hepatitis, drug-induced liver disease, hemochromatosis, α-1-antitrypsin deficiency, Wilson disease, primary biliary cholangitis, autoimmune liver disease, and systemic autoimmune diseases (e.g., systemic lupus erythematosus or rheumatoid arthritis). There were 52 patients with NAFLD, which was defined by an upper limit of alcohol intake of 30 g/day in males and 20 g/day in females [9]. There were 20 patients with alcoholic fatty liver disease (AFLD), which was defined as alcohol intake in excess of the upper limit.
The Human Ethics Review Committee at Toranomon Hospital approved the protocol of the study. Signed informed consent forms were obtained from each of the patients at the time of liver histological diagnosis. The study complied with the International Conference on Harmonization Guidelines for Good Clinical Practice (E6) and the 2013 Declaration of Helsinki.
Diagnosis of liver cancer
The diagnosis of liver cancer in all 258 patients was confirmed by imaging studies, including abdominal ultrasound (US), dynamic computed tomography (CT), and magnetic resonance imaging (MRI). For the 117 patients (45.3%) who underwent surgical resection, the diagnosis of liver cancer was confirmed with histopathological examination. The tumor characteristics were evaluated according to the Barcelona Clinic Liver Cancer (BCLC) staging [10].
Clinical parameters
A normal level of AFP was defined as 10 μg/L or less, and that of PIVKAII was 40 AU/L or less. The Fib-4 index was used as a parameter for the progression of fibrosis and was calculated as follows: [age (year) × AST (IU/L)]/[platelet count (109/L) × √ALT (IU/L)] [11].
Assessment of TERT promoter mutation by wild-type blocking PCR
Our group recently developed a highly sensitive method for the detection of TERT promotor mutation using wild-type blocking PCR (WTB-PCR), combined with Sanger sequencing, and demonstrated its clinical usefulness for early prediction of liver cancer, by measuring TERT C228T in serum cfDNA [12]. The sequencing analysis of WTB-PCR product demonstrated a detection limit in excess of 0.7% Mutant-type DNA in the background of Wild-type DNA [12]. Thus, in the present study we serially examined the relationship between liver cancer and TERT C228T in serum cfDNA by WTB-PCR.
After withdrawal of blood samples, serum was frozen at – 80 °C within 4 h of collection then thawed just before analysis. The genome DNA was extracted from 1,000 μL of serum with QIAamp® Circulating Nucleic Acid Kit (Qiagen, Tokyo), and the nucleotide sequences were determined by direct sequencing. The primers used were TERT promoter F (5´-CAGCGCTGCCTGAAACTC-3´; nucleotides 1,295,151–1,295,168 on chromosome 5) and TERT promoter R2 (5´-GGCCGATTCGACCTCTCT-3´; nucleotides 1,295,528–1,295,511 on chromosome 5). The genome sequence of 378 nucleotides was determined. The 228-LNA (5´-gcccagcccCCTccgggccct-3´; capital letters indicate LNA) was used as the blocking oligonucleotide for TERT promoter at position 228 (TERT228). WTB-PCR master mix was prepared using 12.5 µL 2 × buffer, 5 µL dNTPs, 1 µL forward primer, 1 µL reverse primer, 1 µL blocking oligonucleotide for TERT228, 0.5 µL KOD SYBR® qPCR Mix (Toyobo Co., Osaka, Japan), and 3 µL double-distilled H2O to create a final solution volume of 24 µL per reaction. Of this, 1 µL was used for genomic DNA. First denaturation was performed at 94 ºC for 2 min, and 40 cycles of amplifications were performed as follows: denaturation for 10 s at 98 ºC, annealing of primers for 30 s at 62 ºC followed by 5 s at 72 ºC, extension for 30 s at 68 ºC, and final extension was performed at 68 ºC for 7 min. The PCR-amplified DNA was purified after agarose gel electrophoresis and then used for direct sequencing. The latter was conducted using the dye terminator method. Dideoxynucleotide termination sequencing was performed using the Big Dye® Terminator Cycle Sequencing kit (Life Technologies, Tokyo). We defined TERT C228T “positive” samples as those with mutant peak detected at position 228 (228 T), based on the electropherograms in sequencing [8, 12].
Statistical analysis
Non-parametric tests were used to compare variables between groups, including the chi-squared test, Fisher’s exact probability test, and Mann–Whitney U test. Univariate and multivariate logistic regression analyses were used to determine the independent predictive factors associated with TERT C228T-positive liver cancer. The parameters in Table 1 that indicated strong correlations with other parameters were considered confounding factors and excluded from the univariate and multivariate analyses. Thus, the parameters shown in Tables 2, 3 were used for the analysis of the predictive factors.
Each variable was transformed into categorical data consisting of two simple ordinal numbers for the univariate and multivariate analyses. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were also calculated. All p values less than 0.05 according to a two-tailed test were considered significant. Variables that achieved statistical significance (p < 0.05) or marginal significance (p < 0.10) in the univariate analysis were entered into the multiple logistic regression analysis to identify significant independent factors. All statistical tests were performed with the Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL).
Results
Clinical factors associated with TERT C228T-positive liver cancer
Data from all 258 patients who were confirmed to have a diagnosis of liver cancer with imaging studies were analyzed to identify clinical factors associated with TERT C228T-positive liver cancer. The univariate analysis identified 2 parameters that tended to be or were significantly correlated with TERT C228T-positive liver cancer: body mass index (≥ 25.0 kg/m2, p = 0.047) and etiology (FLD vs. HBV; p = 0.009). Biochemical markers reflecting inflammation were not different according to TERT C228T (AST, p = 0.194; ALT, p = 0.507; Total bilirubin, p = 0.551; and AFP, p = 0.150; Mann–Whitney U test). The multivariate analysis included these factors and identified etiology (FLD vs. HBV; OR 2.346, p = 0.010) as a significant and independent determinant of TERT C228T-positive liver cancer (Table 2).
Histopathological factors associated with TERT C228T-positive liver cancer
Data from 117 patients who underwent surgical resection and were confirmed to have a diagnosis of liver cancer with histopathological examination were analyzed to identify histopathological factors associated with TERT C228T-positive liver cancer. The univariate analysis identified 2 parameters that tended to be or were significantly correlated with TERT C228T-positive liver cancer: vp (presence, p = 0.097) and fibrosis stage (0, 1, 2; p = 0.047). The multivariate analysis that included these factors identified vp (presence; OR 2.472, p = 0.037) and fibrosis stage (0, 1, 2; OR 3.774, p = 0.003) as significant and independent determinants of TERT C228T-positive liver cancer (Table 3).
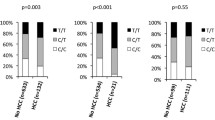
Relationships between etiology of liver cancer and TERT C228T/AFP/PIVKAII
The rates of positive TERT C228T in FLD patients were significantly higher than those of HBV patients (p = 0.009; chi-squared test) and HCV patients (p = 0.017; chi-squared test) (Fig. 1A). The rates of abnormal AFP levels in FLD patients were significantly lower than those of HBV patients (p = 0.001; chi-squared test) and HCV patients (p = 0.012; chi-squared test) (Fig. 1B). The rates of abnormal PIVKAII levels in FLD patients were significantly higher than those of HBV patients (p = 0.020; chi-squared test) and HCV patients (p < 0.001; chi-squared test) (Fig. 1C). Relationships among etiology, TERT C228T/AFP/PIVKAII, and histopathological findings of 117 patients who underwent liver cancer surgical resection, were shown in Table 4.
Relationships between TERT C228T and viral suppression/alcohol intake
The rates of positive TERT C228T were not different between the two groups of HBV (57 patients who did not receive NUCs and 33 patients who achieved viral suppression under NUCs) (p = 0.334; chi-squared test) (Fig. 2A). There were no differences between the three groups of HCV (30 patients who did not receive DAAs, 10 patients who did not achieve SVR by DAAs, and 56 patients who achieved SVR by DAAs) (p = 0.216; chi-squared test) (Fig. 2B). There were also no differences between the two groups of FLD (52 patients who were diagnosed as NAFLD and 20 patients who were diagnosed as AFLD) (p = 0.793; chi-squared test) (Fig. 2C). Interestingly, the rates of positive TERT C228T in HCV patients who achieved SVR were significantly lower than those of FLD patients (p = 0.011; chi-squared test).
Relationships between TERT C228T and viral suppression/alcohol intake. A Rates of positive TERT C228T in HBV, B those in HCV, and C those in FLD according to viral suppression or alcohol intake. AFLD alcoholic fatty liver disease, DAAs direct-acting antivirals, NAFLD non-alcoholic fatty liver disease, NUCs nucleos(t)ide analogues
Relationships between AFP/PIVKAII and viral suppression/alcohol intake
The rates of abnormal AFP levels in HBV patients who did not receive NUCs were significantly higher than those of HBV patients who achieved viral suppression under NUCs (p = 0.046; chi-squared test) (Supplement Fig. 1A). There were significant differences between the three groups of HCV (p < 0.001; chi-squared test). Particularly, AFP levels of HCV patients who did not receive DAAs were significantly higher than those of HCV patients who achieved SVR by DAAs (p = 0.001; chi-squared test) (Supplement Fig. 1B). There were no differences between the two groups of FLD (NAFLD and AFLD) (p = 0.575; chi-squared test) (Supplement Fig. 1C).
The rates of abnormal PIVKAII levels in HBV patients showed no differences between patients who did not receive NUCs and patients who achieved viral suppression under NUCs (p = 0.636; chi-squared test) (Supplement Fig. 2A). There were no differences between the three groups of HCV (p = 0.117; chi-squared test) (Supplement Fig. 2B). There were no differences between the two groups of FLD (NAFLD and AFLD) (p = 0.603; chi-squared test) (Supplement Fig. 2C).
Discussion
Molecular therapies and precision medicine for liver cancer are anticipated. Llovet et al. presented an integrative molecular and immunological classification of liver cancer [13, 14]. From the perspective of etiology, proliferation-class tumors are associated with HBV-related liver cancer, and non-proliferation-class tumors are associated with alcohol and HCV-related liver cancer. HBV-related liver cancer tends to contain the histological features of poorly differentiated HCC and higher frequencies of vascular invasion. Furthermore, HBV-related liver cancer indicates higher AFP levels [13]. Unfortunately, our results could highlight the importance of serum TERT C228T for the detection of non-viral FLD-related liver cancer, but the superiority of TERT C228T could not be compared with AFP or with PIVKAII. Hence, we should require attention to interpretation of the present findings. Further study should be performed to compare the usefulness of three serological markers for the detection of FLD-related liver cancer.
From the perspective of DNA somatic alterations, it is unclear whether there might be a difference among HCV, NAFLD, and alcoholic-related liver cancer. Pinyol et al. reported that rates of positive TERT C228T in NAFLD-related liver cancer were not significantly different from those of HBV/HCV/alcoholic-related liver cancer [15]. However, it is unknown whether the rates of positive TERT C228T might be different among the three etiologies of HBV, HCV, and FLD-related liver cancer. The present results indicated that those of FLD were significantly higher than those of HBV and HCV, while those of NAFLD were not different from those of AFLD. To our knowledge, the present study is the first to highlight the importance of TERT C228T for the detection of non-viral FLD-related liver cancer. The present findings based on the difference of etiology might be useful for the development of molecular therapies and precision medicine for liver cancer. As one limitation of the present study, we could not examine TERT C228T in the precancerous serum samples without liver cancer. Our previous report showed the rates of positive TERT C228T were 4.8% in serum samples of NAFLD without liver cancer (Supplement Table) [8]. Other previous report indicated positive rates of 8.6% in plasma samples of cirrhosis without liver cancer, including the etiologies of HBV, HCV, and FLD [16]. Further study according to the etiology should be performed to investigate the difference in the rates of positive TERT C228T in precancerous stage without liver cancer.
Pfister et al. recently reported that NAFLD-related liver cancer might be less responsive to immune checkpoint inhibitors, which is probably due to NAFLD-related aberrant T cell activation causing tissue damage that leads to impaired immune surveillance [6]. Compared to other etiologies, NAFLD-related liver cancer shows a significantly higher prevalence of an immunosuppressive cancer field [15]. This evidence provides a rationale for stratification of patients with liver cancer according to the underlying etiology in studies of immunotherapy as a primary or adjuvant treatment. One limitation of the present study is the lack of analysis of comparison between tissue of liver cancer and the corresponding cfDNA. Previous report indicated that TERT promoter mutations in tissue of cirrhosis correlate with the rate of hepatocarcinogenesis, with mutations identified in 6% of low grade dysplastic nodules, 19% of high grade dysplastic nodules, and 61% of early liver cancer [17]. However, it is still unclear whether mutations of cfDNA might reflect those of tissue. Another limitation of the present study is that the difference in Wnt/CTNNB1 mutations, apart from TERT promoter mutations, could not be investigated according to the etiology of liver cancer. Further studies should be performed to develop molecular therapies and precision medicine for liver cancer based on DNA somatic alterations.
To our knowledge, the present study is the first to investigate the relationships between TERT C228T and viral suppression/alcohol intake. Basically, neither viral suppression nor alcohol intake significantly affected the rates of positive TERT C228T. On the other hand, viral suppression of HBV and HCV significantly reduced AFP levels. One reason for these discrepant results might be that AFP levels reflect not only the potential of carcinogenesis, but also higher levels of inflammation [18,19,20]. Hence, the present results also showed that TERT C228T was a tumor marker that might not be influenced by inflammation. Interestingly, the rates of positive TERT C228T in HCV patients who achieved viral clearance were significantly lower than those of FLD patients. This finding indicates that the two groups of FLD and HCV with SVR should be classified in precision medicine for liver cancer based on DNA somatic alterations.
In conclusion, our results highlight the importance of serum TERT C228T for the detection of non-viral FLD-related liver cancer. TERT C228T is a tumor marker that might not be influenced by inflammation. Early diagnosis and treatment based on DNA somatic alterations might improve the outcome of non-viral FLD patients who develop liver cancer.
Availability of data and materials
The datasets generated or analyzed in the present study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- AFLD:
-
Alcoholic fatty liver disease
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona Clinic Liver Cancer
- CCC:
-
Cholangiocellular carcinoma
- cfDNA:
-
Cell-free DNA
- DAAs:
-
Direct acting antivirals
- FLD:
-
Fatty liver disease
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- LNA:
-
Locked nucleic acid
- MT:
-
Mutant-type
- NUCs:
-
Nucleos(t)ide analogues
- PCR:
-
Polymerase chain reaction
- PIVKAII:
-
Des-γ-carboxyprothrombin
- SVR:
-
Sustained virological response
- TERT :
-
Telomerase reverse transcriptase
- WTB:
-
Wild-type blocking
References
Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–1273
Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511
Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341
Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218
Chen YL, Jeng YM, Chang CN, Lee HJ, Hsu HC, Lai PL, et al. TERT promoter mutation in resectable hepatocellular carcinomas: a strong association with hepatitis C infection and absence of hepatitis B infection. Int J Surg. 2014;12:659–665
Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456
Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–2023
Akuta N, Kawamura Y, Kobayashi M, Arase Y, Saitoh S, Fujiyama S, et al. TERT promoter mutation in serum cell-free DNA is a diagnostic marker of primary hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Oncology. 2021;99:114–123
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325
Akuta N, Suzuki F, Kobayashi M, Fujiyama S, Kawamura Y, Sezaki H, et al. Detection of TERT promoter mutation in serum cell-free DNA using wild-type blocking PCR combined with Sanger sequencing in hepatocellular carcinoma. J Med Virol. 2020;92:3604–3608
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6
Pinyol R, Torrecilla S, Wang H, Montironi C, Piqué-Gili M, Torres-Martin M, et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75:865–878
Jiao J, Watt GP, Stevenson HL, Calderone TL, Fisher-Hoch SP, Ye Y, et al. Telomerase reverse transcriptase mutations in plasma DNA in patients with hepatocellular carcinoma or cirrhosis: Prevalence and risk factors. Hepatol Commun. 2018;2:718–731
Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983–1992
Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, et al. Clinical, virological, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240–244
Stein DF, Myaing M. Normalization of markedly elevated α-fetoprotein in a virologic nonresponder with HCV-related cirrhosis. Dig Dis Sci. 2002;47:1686–2690
Hu KQ, Kyulo N, Lim N, Elhazin B, Hillebrand DJ, Bock T. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860–865
Acknowledgements
The authors thank the following pathologists for their assistance in histopathological diagnosis: Keiichi Kinowaki, M.D., Department of Pathology, Toranomon Hospital; Fukuo Kondo, M.D., Department of Pathology, Teikyo University School of Medicine; Toshio Fukusato, M.D., Department of Pathology, Teikyo University School of Medicine; and Takeshi Fujii, M.D., Department of Pathology, Toranomon Hospital. The authors also thank Rie Mineta and Kenichi Tadokoro for their research assistance.
Funding
This study was supported in part by Grant-in-Aid from the Japan Agency for Medical Research and Development (JP21fk0210058, JP21fk0210065, JP21fk0210073, JP21fk0210090).
Author information
Authors and Affiliations
Contributions
NA, YK, FS, MK (Mariko Kobayashi), SS, YA, NM, SF, HS, TH, MK (Masahiro Kobayashi), YS, KI, and HK contributed to this work. NA, YK, and YA analyzed the data. NA wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
(1) Hiromitsu Kumada has received honoraria from Gilead Sciences, AbbVie Inc., Eisai Co., Ltd., and Dainippon Sumitomo Pharma. (2) Yusuke Kawamura has received honoraria from Eisai Co., Ltd. Norio Akuta, Yusuke Kawamura, Fumitaka Suzuki, Mariko Kobayashi, Yasuji Arase, Satoshi Saitoh, Nozomu Muraishi, Shunichiro Fujiyama, Hitomi Sezaki, Tetsuya Hosaka, Masahiro Kobayashi, Yoshiyuki Suzuki, Kenji Ikeda and Hiromitsu Kumada declare that they have no conflicts of interest.
Ethical approval and consent to participate
The study protocol was approved by the Human Ethics Review Committee at Toranomon Hospital, and each patient provided a signed informed consent form at the time of liver histological diagnosis. The study was conducted in compliance with the International Conference on Harmonization Guidelines for Good Clinical Practice (E6) and the 2013 Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2022_10313_MOESM1_ESM.tif
Supplement Figure 1. Relationships between AFP levels and viral suppression/alcohol intake. (A) Rates of abnormal AFP levels in HBV, (B) those in HCV, and (C) those in FLD according to viral suppression or alcohol intake. Normal level of AFP is defined as 10 μg/L or less. AFLD: alcoholic fatty liver disease, DAAs: direct-acting antivirals, NAFLD: non-alcoholic fatty liver disease: NUCs: nucleos(t)ide analogues
12072_2022_10313_MOESM2_ESM.tif
Supplement Figure 2. Relationships between PIVKAII levels and viral suppression/alcohol intake. (A) Rates of abnormal PIVKAII levels in HBV, (B) those in HCV, and (C) those in FLD according to viral suppression or alcohol intake. Normal level of PIVKAII is 40 AU/L or less. AFLD: alcoholic fatty liver disease, DAAs: direct-acting antivirals, NAFLD: non-alcoholic fatty liver disease: NUCs: nucleos(t)ide analogues
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akuta, N., Kawamura, Y., Suzuki, F. et al. Serum TERT C228T is an important predictor of non-viral liver cancer with fatty liver disease. Hepatol Int 16, 412–422 (2022). https://doi.org/10.1007/s12072-022-10313-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10313-y