Abstract
Background
MicroRNAs have added a new dimension to our understanding of liver cirrhosis (LC) and associated processes like the activation of hepatic stellate cells (HSCs).
Methods
Serum samples were collected from 40 LC patients and 30 healthy donors. CCl4-induced LC mouse model in vivo and in vitro human HSC LX-2 and murine HSC JS-1 cells were researched.
Results
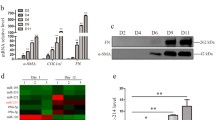
The levels of serum microRNA (miR)-744 is inversely correlated with the severity of LC and is a reliable biomarker of LC. In CCl4-induced LC model, the abundance of miR-744 was reduced in both sera and livers compared with sham controls. Importantly, increasing miR-744 abundance with synthetic miR-744 Agomir alleviated liver fibrosis, a critical component of LC, while reducing miR-744 with Antagomir exacerbated it. To elucidate molecular mechanism underlying the suppressive role of miR-744 in LC, we observed that miR-744 and transforming growth factor β1 (TGFβ1) are inversely correlated in LC patients’ sera as well as sera/livers from CCl4-induced LC mice. We demonstrated that miR-744 Agomir downregulated the expression of TGFβ1 and further confirmed that TGFβ1 mRNA was a bona fide miR-744 target in HSCs. Moreover, miR-744 Agomir reduced the degree of F-actin formation and cell proliferation while miR-744 Antagomir promoted these events, suggesting that miR-744 is a negative regulator of HSC activation.
Conclusions
MiR-744-led suppression in HSC activation is most likely through TGFβ1 because exogenous TGFβ1 nearly negated miR-744 Agomir’s action. This study suggests that reduction of miR-744 is a reliable biomarker for LC and miR-744/TGFβ1 relationship is a key regulator of LC.






Similar content being viewed by others
Abbreviations
- LC:
-
Liver cirrhosis
- HSC:
-
Hepatic stellate cell
- ECM:
-
Extracellular matrix
- miRNA:
-
MicroRNA
- UTR:
-
Untranslated region
- TGFβ:
-
Transforming growth factor β
- EMT:
-
Epithelial-to-mesenchymal transition
- CHB:
-
Chronic hepatitis B
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- SUTCM:
-
Shanghai University of Traditional Chinese Medicine
- CCl4 :
-
Carbon tetrachloride
- PBS:
-
Phosphate buffered saline
- ANOVA:
-
Analysis of variance
- ROC:
-
Receiver–operator characteristic
References
Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol 2009;25:223–229
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655–1669
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297
Schueller F, Roy S, Vucur M, Trautwein C, Luedde T, Roderburg C. The role of miRNAs in the pathophysiology of liver diseases and toxicity. Int J Mol Sci 2018;19:261
Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett 2009;583:759–766
Sun X, He Y, Ma TT, Huang C, Zhang L, Li J. Participation of miR-200a in TGF-beta1-mediated hepatic stellate cell activation. Mol Cell Biochem 2014;388:11–23
Zhang H, Li QY, Guo ZZ, Guan Y, Du J, Lu YY, et al. Serum levels of microRNAs can specifically predict liver injury of chronic hepatitis B. World J Gastroenterol 2012;18:5188–5196
Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis 2001;21:427–436
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci 2002;7:d793–d807
Shen H, Huang G, Hadi M, Choy P, Zhang M, Minuk GY, et al. Transforming growth factor-beta1 downregulation of Smad1 gene expression in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2003;285:G539–G546
Cheng K, Yang N, Mahato RI. TGF-beta1 gene silencing for treating liver fibrosis. Mol Pharm 2009;6:772–779
Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA 1999;96:2345–2349
Chinese Society of Hepatology and Chinese Society of Infectious Diseases CMA. The guideline of prevention and treatment for chronic hepatitis B (2010 version). J Clin Hepatol 2011;27:I–XVI
Yi Y, Zhao Y, Li C, Zhang L, Huang H, Li Y, et al. RAID v2.0: an updated resource of RNA-associated interactions across organisms. Nucleic Acids Res 2017;45:D115–D118
Blin K, Dieterich C, Wurmus R, Rajewsky N, Landthaler M, Akalin A. DoRiNA 2.0–upgrading the doRiNA database of RNA interactions in post-transcriptional regulation. Nucleic Acids Res 2015;43:D160–D167
Bhattacharya A, Ziebarth JD, Cui Y. PolymiRTS Database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res 2014;42:D86–D91
Xin X, Zhang Y, Liu X, Xin H, Cao Y, Geng M. MicroRNA in hepatic fibrosis and cirrhosis. Front Biosci (Landmark Ed). 2014;19:1418–1424
Kwiecinski M, Noetel A, Elfimova N, Trebicka J, Schievenbusch S, Strack I, et al. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS One 2011;6:e24568
Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011;53:209–218
Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 2011;45:287–294
Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, et al. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One 2012;7:e33608
Kubiczkova L, Kryukov F, Slaby O, Dementyeva E, Jarkovsky J, Nekvindova J, et al. Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica 2014;99:511–518
Mi QS, Weiland M, Qi RQ, Gao XH, Poisson LM, Zhou L. Identification of mouse serum miRNA endogenous references by global gene expression profiles. PLoS One 2012;7:e31278
Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350–1358
Martin J, Jenkins RH, Bennagi R, Krupa A, Phillips AO, Bowen T, et al. Post-transcriptional regulation of Transforming Growth Factor Beta-1 by microRNA-744. PLoS One 2011;6:e25044
Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 2007;46:48–57
Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol 2016;22:10512–10522
Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J Hepatol 2009;50:766–778
Zheng J, Lin Z, Dong P, Lu Z, Gao S, Chen X, et al. Activation of hepatic stellate cells is suppressed by microRNA-150. Int J Mol Med 2013;32:17–24
Li F, Ma N, Zhao R, Wu G, Zhang Y, Qiao Y, et al. Overexpression of miR-483-5p/3p cooperate to inhibit mouse liver fibrosis by suppressing the TGF-beta stimulated HSCs in transgenic mice. J Cell Mol Med 2014;18:966–974
Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 81530101), NIH CA187152 and CA222467.
Author information
Authors and Affiliations
Contributions
SR, SH and PL designed research. SR, JC, XL, WF and XZ performed the experiments and analyzed the data. YM, HZ, MS and CL contributed experimental materials or provided helpful suggestions. SR, JC, SH and PL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Shuang Ren, Jiamei Chen, Qinglan Wang, Xuewei Li, Ying Xu, Xiao Zhang, Yongping Mu, Hua Zhang, Shuang Huang, Ping Liu have no confict of interest to declare.
Ethics approval
Ethics approval all procedures performed in studies involving human participants and animals were in accordance with the ethical standards of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine. The entire study was approved by Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
Informed consent
Informed consent was collected from patients to approve utilization of their samples for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, S., Chen, J., Wang, Q. et al. MicroRNA-744/transforming growth factor β1 relationship regulates liver cirrhosis. Hepatol Int 13, 814–825 (2019). https://doi.org/10.1007/s12072-019-09993-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09993-w