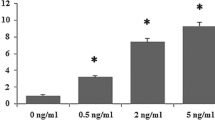
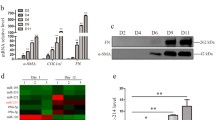
Abstract
Hepatic stellate cell (HSC) activation is a pivotal event in the initiation and progression of hepatic fibrosis since it mediates transforming growth factor beta 1 (TGF-β1)-driven extracellular matrix (ECM) deposition. MicroRNAs (miRNAs), small non-coding RNAs modulating messenger RNA (mRNA) and protein expression, have emerged as key factors to regulate cell proliferation, differentiation, and apoptosis. Although the function of miR-200a has been discussed in many cancers and fibrotic diseases, its role in hepatic fibrosis is still poorly understood. The aim of this study is to investigate whether miR-200a could attenuate hepatic fibrosis partly through Wnt/β-catenin and TGF-β-dependant mechanisms. Our study found that the expression of endogenous miR-200a was decreased in vitro in TGF-β1-induced HSC activation as well as in vivo in CCl4-induced rat liver fibrosis. Overexpression of miR-200a significantly inhibited α-SMA activity and further affected the proliferation of TGF-β1-dependent activation of HSC. In addition, we identified β-catenin and TGF-β2 as two functional downstream targets for miR-200a. Interestingly, miR-200a specifically suppressed β-catenin in the protein level, whereas miR-200a-mediated suppression of TGF-β2 was shown on both mRNA and protein levels. Our results revealed the critical regulatory role of miR-200a in HSC activation and implied miR-200a as a potential candidate for therapy by deregulation of Wnt/β-catenin and TGFβ signaling pathways, at least in part, via decreasing the expression of β-catenin and TGF-β2.







Similar content being viewed by others
Abbreviations
- HSC:
-
Hepatic stellate cell
- ECM:
-
Extracellular matrix
- α-SMA:
-
α-Smooth muscle actin
- TGF-β:
-
Transforming growth factor-β
- β-catenin:
-
Cadherin-associated protein beta
- 3′-UTR:
-
3′-Untranslated region
- PBS:
-
Phosphate-buffered saline
- SDS:
-
Sodium dodecyl sulfate
- Wt:
-
Wild type
- ZEB:
-
Zinc-finger E-box-binding homeobox
- One-step qRT-PCR:
-
One-step quantitative real-time PCR
References
Friedman SL, Maher JJ, Bissell DM (2000) Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology 32(6):1403–1408. doi:10.1053/jhep.2000.20243
Wells RG (2005) The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol 39(4 Suppl 2):S158–S161
Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88(1):125–172. doi:10.1152/physrev.00013.2007
Lakner AM, Steuerwald NM, Walling TL, Ghosh S, Li T, McKillop IH, Russo MW, Bonkovsky HL, Schrum LW (2012) Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology 56(1):300–310. doi:10.1002/hep.25613
Gressner AM (1996) Mediators of hepatic fibrogenesis. Hepatogastroenterology 43(7):92–103
Gressner AM, Weiskirchen R (2006) Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 10(1):76–99
Kingsley DM (1994) The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8(2):133–146
Carrington LM, Albon J, Anderson I, Kamma C, Boulton M (2006) Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci 47(5):1886–1894. doi:10.1167/iovs.05-0635
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61(4):337–346
Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H (2008) Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 294(1):G39–G49. doi:10.1152/ajpgi.00263.2007
Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12(2):99–110. doi:10.1038/nrg2936
Guo CJ, Pan Q, Jiang B, Chen GY, Li DG (2009) Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis 14(11):1331–1340. doi:10.1007/s10495-009-0401-3
Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S (2009) Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett 583(4):759–766. doi:10.1016/j.febslet.2009.01.034
Sekiya Y, Ogawa T, Iizuka M, Yoshizato K, Ikeda K, Kawada N (2011) Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol 226(10):2535–2542. doi:10.1002/jcp.22598
Chen C, Wu CQ, Zhang ZQ, Yao DK, Zhu L (2011) Loss of expression of miR-335 is implicated in hepatic stellate cell migration and activation. Exp Cell Res 317(12):1714–1725. doi:10.1016/j.yexcr.2011.05.001
Wang B, Li W, Guo K, Xiao Y, Wang Y, Fan J (2012) miR-181b promotes hepatic stellate cells proliferation by targeting p27 and is elevated in the serum of cirrhosis patients. Biochem Biophys Res Commun 421(1):4–8. doi:10.1016/j.bbrc.2012.03.025
Venugopal SK, Jiang J, Kim TH, Li Y, Wang SS, Torok NJ, Wu J, Zern MA (2010) Liver fibrosis causes downregulation of miRNA-150 and miRNA-194 in hepatic stellate cells, and their overexpression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol 298(1):G101–G106. doi:10.1152/ajpgi.00220.2009
He Y, Huang C, Sun X, Long XR, Lv XW, Li J (2012) MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal 24(10):1923–1930. doi:10.1016/j.cellsig.2012.06.003
Kwiecinski M, Elfimova N, Noetel A, Tox U, Steffen HM, Hacker U, Nischt R, Dienes HP, Odenthal M (2012) Expression of platelet-derived growth factor-C and insulin-like growth factor I in hepatic stellate cells is inhibited by miR-29. Lab Invest 92(7):978–987. doi:10.1038/labinvest.2012.70
Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G (2012) Participation of miR-200 in pulmonary fibrosis. Am J Pathol 180(2):484–493. doi:10.1016/j.ajpath.2011.10.005
Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME, Kantharidis P (2011) miR-200a prevents renal fibrogenesis through repression of TGF-beta2 expression. Diabetes 60(1):280–287. doi:10.2337/db10-0892
Lafyatis R (2006) Targeting fibrosis in systemic sclerosis. Endocr Metab Immune Disord Drug Targets 6(4):395–400
Schuppan D, Ruehl M, Somasundaram R, Hahn EG (2001) Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis 21(3):351–372. doi:10.1055/s-2001-17556
Shimada H, Staten NR, Rajagopalan LE (2011) TGF-beta1 mediated activation of Rho kinase induces TGF-beta2 and endothelin-1 expression in human hepatic stellate cells. J Hepatol 54(3):521–528. doi:10.1016/j.jhep.2010.07.026
Chau BN, Brenner DA (2011) What goes up must come down: the emerging role of microRNA in fibrosis. Hepatology 53(1):4–6. doi:10.1002/hep.24071
Kalluri R, Weinberg RA (2009) The basics of epithelial–mesenchymal transition. J Clin Invest 119(6):1420–1428. doi:10.1172/JCI39104
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. doi:10.1016/j.cell.2009.11.007
Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ (2011) An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 22(10):1686–1698. doi:10.1091/mbc.E11-02-0103
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L, Tan R, Wen P, Yang J (2012) The miR-200 family regulates TGF-beta1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol 302(3):F369–F379. doi:10.1152/ajprenal.00268.2011
Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, Tajima A, Kosaka N, Ochiya T, Shimotohno K (2011) The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One 6(1):e16081. doi:10.1371/journal.pone.0016081
Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP (2004) Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy 34(3):437–444
Serpero L, Petecchia L, Sabatini F, Giuliani M, Silvestri M, Di Blasi P, Rossi GA (2006) The effect of transforming growth factor (TGF)-beta1 and (TGF)-beta2 on nasal polyp fibroblast activities involved upper airway remodeling: modulation by fluticasone propionate. Immunol Lett 105(1):61–67. doi:10.1016/j.imlet.2006.01.003
Lee SY, Chuang JH, Huang CC, Chou MH, Wu CL, Chen CM, Hsieh CS, Chen CL (2004) Identification of transforming growth factors actively transcribed during the progress of liver fibrosis in biliary atresia. J Pediatr Surg 39(5):702–708
Jiang F, Parsons CJ, Stefanovic B (2006) Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol 45(3):401–409. doi:10.1016/j.jhep.2006.03.016
Nejak-Bowen K, Monga SP (2008) Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis 4(2):92–99
Medici D, Hay ED, Olsen BR (2008) Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell 19(11):4875–4887. doi:10.1091/mbc.E08-05-0506
Wang Y, Sun Z, Qiu X, Li Y, Qin J, Han X (2009) Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 390(4):1309–1314. doi:10.1016/j.bbrc.2009.10.143
Saydam O, Shen Y, Wurdinger T, Senol O, Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens RM, Fraefel C, Gusella JF, Krichevsky AM, Breakefield XO (2009) Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol 29(21):5923–5940. doi:10.1128/MCB.00332-09
Huang H, He X (2008) Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20(2):119–125. doi:10.1016/j.ceb.2008.01.009
Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287(5458):1606–1609
Polakis P (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17(1):45–51. doi:10.1016/j.gde.2006.12.007
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127(3):469–480. doi:10.1016/j.cell.2006.10.018
Acknowledgments
This project was supported by the National Science Foundation of China (Nos: 81072686, 81273526, and 81202978) and the Natural Science Foundation of Anhui Province (KJ2010A178).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, X., He, Y., Ma, TT. et al. Participation of miR-200a in TGF-β1-mediated hepatic stellate cell activation. Mol Cell Biochem 388, 11–23 (2014). https://doi.org/10.1007/s11010-013-1895-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1895-0