Abstract
The aim of this study was to find out the association of sinonasal candidiasis and Covid-19 infection. A prospective observational study was conducted at a tertiary care centre from April to September 2021, involving all patients with invasive candidiasis of the paranasal sinuses having a history of Covid-19 infection. A total of 18 patients of covid associated sinonasal candidiasis among the 475 cases of fungal rhinosinusitis were studied. All patients had involvement of nose and sinuses and 2 patients had orbital involvement with no loss of vision, while 3 had intracranial extensions and 1 had pulmonary involvement. Mandible was involved in 1 patient alone, while the maxilla and palate were involved in 5 patients. 15 patients were hypertensive, 12 diabetics and 1 had aplastic anaemia. Cultures showed that 8 patients had C. parapsilosis, 5 had C. albicans, 3 had C. tropicalis and 2 had mixed fungal infections. All patients underwent surgical debridement and antifungal administration. They were followed up for a minimum of 3 months. There was only one mortality (with aplastic anaemia), rest 17 were disease free at the time of writing this article. This is perhaps the first case series of post covid sinonasal candidiasis in the world. Invasive sinonasal candidiasis is a newer sequela of COVID-19 infection. Uncontrolled diabetes and over-zealous use of steroids at the time of Covid-19 are few of the known risk factors. Early surgical intervention and anti-fungal treatment should be sought for management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Covid-19 is a new entity among the spectrum of respiratory infections caused by SARS-CoV-2 or Severe Acute Respiratory Syndrome Corona Virus-2. The infection first detected in December 2019 in Wuhan, China spread worldwide within a short span and with every passing day had newer alterations in its pathophysiology, management, sequelae and complications [1]. We saw a gradual rise in fungal sinusitis associated with covid infection in the first peak of Covid-19 [2]. These co-infections rose to a sudden surge with the second peak of Covid-19 in May 2021 in India and led to an epidemic.
Invasive fungal sinusitis is a life-threatening infection that typically affects immunocompromised individuals with an impaired neutrophilic response. Patients can include those with uncontrolled diabetes mellitus, acquired immunodeficiency syndrome, iatrogenic immunosuppression and haematological malignancies, and those having undergone organ transplantation [3]. Covid associated mucormycosis has also been widely described and a study by Sharma et al. talked about this even in the first wave of Covid 19 [4]. Invasive rhino-orbito-cerebral candidiasis on the other hand, has not been described much. We attempt to throw light on this, with our experience of 18 cases of sinonasal candidiasis over a period of 6 months.
Materials and Methods
A prospective observational study was undertaken at Sawai Man Singh Medical College and Hospital, Jaipur, India, over a period of 6 months, from April to September 2021. All patients with invasive rhino-orbito-cerebral candidiasis who had recovered from coronavirus infection, and with any 2 of the following 3 screening procedures positive for invasive FRS-nasal/palatal biopsy, KOH mount and Gadolinium enhanced MRI scans with fat suppression and diffusion weighted imaging, were included in the study. All patients underwent surgical debridement, along with control of immunocompromised status and intravenous antifungal administration. The details of presentation, predisposing history, imaging findings, co-morbidities, management details, and follow-up information were recorded and analysed.
Results
Of the 800 patients screened for FRS post Covid-19, a total of 475 cases of covid associated invasive fungal sinusitis were treated at the institute. Of these, 405 had mucor, 40 had aspergillus, 16 had candida and 12 had mucor with aspergillus, 2 had mucor with candida in covid associated invasive FRS (Table 2). Of the total 18 patients with invasive sinonasal candidiasis, 5 were females and 13 males. Age group was between 5 years to 68 years (mean 50.1 years). All patients had involvement of nose and one or more paranasal sinuses, 2 had orbital involvement (but with no loss of vision), 3 had intracranial extensions and 1 had pulmonary involvement (Tables 1, 2).
All patients had one or more comorbidities: 15 patients were hypertensive, 12 diabetics and 1 had aplastic anaemia. The patient with aplastic anaemia had significant neutropenia and was awaiting bone marrow transplant. 14 patients had received oxygen supplementation and oral or intravenous steroids at the time of covid-19 illness, but none were admitted in ICU.
KOH staining and fungal culture showed various species of Candida. 8 patients had C. parapsilosis, 5 had C. albicans, 3 had C. tropicalis and 2 had mixed fungal infections (Table 3).
All 18 patients had recovered from covid-19 at least 14 days before developing symptoms of fungal disease.
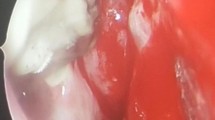
All the patients were surgically debrided and treated with antifungals (echinocandins and azoles) and are being followed up. Intraoperatively the disease was cleared from involved areas, however contrary to findings in mucormycosis, there was no necrotic tissue and the vascularity was more than usual (Fig. 1). Over a follow up of minimum 3 months there was only one mortality. He was having aplastic anaemia with significant neutropenia and cerebral involvement.
Discussion
Covid associated mucormycosis is a known entity, however other species of fungus can also be involved in opportunistic infections after Covid-19. This study deals with such patients of sinonasal candidiasis and is perhaps the first case series in published English literature. Similar to the SARS-CoV and Middle East Respiratory Syndrome (MERS), SARS-CoV-2 may also cause lower respiratory tract infection and lead to acute respiratory distress syndrome(ARDS) [5]. Previous research has demonstrated similarities in prevalence and biological and clinical characteristics of SARS-CoV and SARS-CoV-2 which belong to the same species [6]. During the widespread SARS-CoV infection of 2003, the incidence of fungal infection was 14.8–27%, and it was the main cause of death for severe ARDS patients that is 25–73.7% of all deaths [7, 8].
Prevalence of FRS prior to Covid-19 era was reported at 0.11% of population [9], but post the onset of Covid-19 pandemic, very limited data is available on the prevalence of FRS with Covid-19 [10]. A study reported an incidence of 26.7% for invasive fungal infections among 135 adults with Covid-19 infection [11]. Song et al. concluded that most patients affected by or recovered from Covid-19 are at increased risk of developing invasive fungal diseases when he studied the association between Covid-19 and invasive fungal sinusitis in April 2020, and devised his management algorithm [12]. A recent review showed that 8% of coronavirus-positive or recovered patients had secondary bacterial or fungal infections during hospital admission, under the cover of widespread use of broad spectrum antibiotics and steroids [13]. The second peak of covid-19 with delta strain in 2021 in India, led to a myriad of manifestations and complications and a rarely encountered entity of invasive sinonasal candidiasis.
At least 15 distinct candidial spp. are known to cause human diseases, but the majority of invasive infections are caused by C. albicans, C. glabrata, C. tropicalis, C. parapsilosis and C. krusei [14,15,16]. C. albicans has been encountered most commonly worldwide. Non-albicans candidemia is rare [16]. In India, C. parapsilosis and/or C. tropicalis are much more frequently encountered than C. glabrata [17, 18]. In our study, we found 8 cases of C. parapsilosis, 5 of C. albicans and 2 of C. tropicalis. Candida spp. are common commensals in the skin, gut microbiota in almost 60% of healthy individuals [19]. Further, any break in the cutaneous and gastrointestinal barriers, increased or abnormal colonization and a combined local or generalized defect in host defences promote an invasive disease [19]. Three major conditions predispose to human invasive candidial infections i.e. long-term and/or repeated use of broad-spectrum antibiotics (as this depletes commensals in the gut which release anti-Candida spp. protective factors from the mucosa), breach of the gastrointestinal and cutaneous barriers by cytotoxic chemotherapy-induced mucositis and the third factor is iatrogenic immunosuppression, such as chemotherapy-induced neutropenia or corticosteroid therapy [14, 15].
Besides the diffuse alveolar damage with severe inflammatory exudation, Covid-19 patients always have immunosuppression following a decrease in CD4 T and CD8 T cells, indicating their susceptibility to fungal co-infections [12, 20]. Neutropenia has been well-established as a risk factor for development of the invasive infection and a cause of mortality in humans [21, 22]. In contrast to mucosal candidiasis, during invasive Candida spp. infection immunity relies on myeloid phagocytes (neutrophils, monocytes, macrophages and dendritic cells) and not on lymphocytes [21, 23, 24]. Extensive use of steroids in Covid-19 management can also suppress immunity, allowing opportunistic fungal infections to colonise [4], as reiterated in our study. Clinically, invasive sinonasal candidiasis presents similar to complicated sinusitis, with atypical signs and symptoms like nasal (nasal blockade, crusting, proptosis, facial pain), orbital (oedema, ptosis, chemosis, and even ophthalmoplegia with headache and fever) and neurological, if intracranial extension is present [25, 26]. We echo the same findings in our study. Non-contrast computed tomography scan of the paranasal sinuses is usually the first investigation of choice (Figs. 2 and 3), while gadolinium-enhanced magnetic resonance imaging (Fig. 4) is resorted to when extrasinus extension is suspected [4]. Histological features in candidiasis is (Fig. 5) common to all fungal infections-mycotic infiltration of blood vessels, vasculitis with thrombosis, tissue infarction, haemorrhage and acute neutrophilic infiltrate [27]. Since no clinical signs or symptoms are specific for invasive candidiasis, clinicians have to rely on fungal cultures, histopathological examination and empirical evidence in the setting of ICU, each of which have a low sensitivity [28,29,30]. Thus, timely diagnosis of invasive candidiasis is the key to ensure a favourable outcome. In fact, a delay of 1–2 days in initiation of effective antifungal therapy has been associated with a doubling of mortality [28, 29].
Based on the guidelines by Infectious Diseases Society of America (IDSA) 2016 and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2018, a protocol of initiation therapy with echinocandins and a step down with azoles is being followed for invasive candidiasis [31,32,33]. In our institute, we used echinocandins and azoles in all these patients. Nasal endoscopy at every fortnight with MRI scans at 3 months for prognosis, followed by continuation or stepdown of antifungals was done.
Conclusion
Newer and long-term manifestations of the Covid-19 infection are cropping up. Its association with invasive candidiasis in the current setting is dangerous and should be duly given serious consideration. Uncontrolled diabetes, over-zealous use of steroids, ventilator assisted respiration, central venous catheter, prolonged ICU admissions in association with COVID-19 infection, are few of the main factors aggravating the illness, and must be properly checked. Early surgical intervention, control of immunocompromised status and intravenous antifungal treatment should be the aim of management in such cases.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Wuhan City Health Committee (2019) Wuhan Municipal Health and Health Commission’s briefing on the current pneumonia epidemic situation in our city 2019. In: http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989. Accessed 14 Jan 2020
Panda NK, Sharma SC, Chakrabarti A, Mann SB (1998) Paranasal sinus mycoses in north India. Mycoses 41(7–8):281–6. https://doi.org/10.1111/j.1439-0507.1998.tb00339.x
DeShazo RD (1998) Fungal sinusitis. Am J Med Sci 316:39–44
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T (2021) Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol 135(5):442–447
Wang Y, Wang Y, Chen Y, Qin Q (2020) Unique epidemiological and clinical fea- tures of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 92:568–576
Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S et al (2020) The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and big- gest global health threats: what lessons have we learned? Int J Epidemiol 49:717–726
Yin CH, Wang C, Tang Z, Zhang SW, Wang BS (2004) Clinical analysis of 146 patients with critical severe acute respiratory syndrome in Beijing areas. Clin J Emerg Med 1:12–14
Li CS, Pan SF (2003) Analysis and causation discussion of 185 severe acute respiratory syndrome dead cases. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 15:582–584 ([in Chinese])
Chakrabarti A, Rudramurthy SM, Panda N, Das A, Singh A (2015) Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses 58(5):294–302. https://doi.org/10.1111/myc.12314
Roudbary M, Kumar S, Kumar A, Cernáková L, Nikoomanesh F, Rodrigues CF (2021) Overview on the prevalence of fungal infections, immune response, and microbiome role in COVID-19 patients. J Fungi 7:720
White L, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S et al (2020) A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis 12:98
Song G, Liang G, Liu W (2020) Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia 185:599–606
Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M et al (2020) Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 71:2459–2468
McCarty TP, Pappas PG (2016) Invasive candidiasis. J Infect Dis Clin North Am 30:103–124
Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. N Engl J Med 373:1445–1456
Wisplinghoff H et al (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–176
Castanheira M, Messer SA, Rhomberg PR, Pfaller MA (2016) Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRy antifungal surveillance program. Diagn Microbiol Infect Dis 85:200–204
Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M (2011) Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY antimicrobial surveillance program J. Clin Microbiol 49:396–399
Pappas P, Lionakis M, Arendrup M et al (2018) Invasive candidiasis. Nat Rev Dis Primers 4:18026
Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A et al (2020) Clinical character- istics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 80:388–393
Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL (2015) Immune defence against Candida fungal infections. Nat Rev Immunol 15:630–642
Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Ralph ZJ (2020) Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med 30:100971
Lionakis MS, Netea MG (2013) Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog 9:e1003079
Lionakis MS, Netea MG, Holland SM (2014) Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med 4:a019638
Scheckenbach K, Cornely O, Hoffmann TK, Engers R, Bier H, Chaker A et al (2010) Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx 37:322–328
Vairaktaris E, Moschos MM, Vassiliou S, Baltatzis S, Kalimeras E, Avgoustidis D et al (2009) Orbital cellulitis, orbital subperiosteal and intraorbital abscess. Report of three cases and review of the literature. J Craniomaxillofac Surg 37:132–136
DeShazo RD, Chapin K, Swain RE (1997) Fungal sinusitis. N Engl J Med 337:254–259
Morrell M, Fraser VJ, Kollef MH (2005) Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645
Garey KW et al (2006) Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31
Shobana B et al (2019) Saudi J Pathol Microbiol March 4(3):201–209
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ (2018) Invasive candidiasis. Nat Rev Dis Primers 11(4):18026
Pappas PG et al (2016) Clinical practice Guideline for the management of Candidiasis: update by the infectious Diseases Society of America. Clin Infect Dis 62:1–50
Cornely OA et al (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18:19–37
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare relevant to the content of this article.
Ethics Approval
Not Applicable.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The participant has consented to the submission of the case report to the journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhandari, S., Agarwal, S., Bhargava, S. et al. Post Covid-19 Sinonasal Candidiasis: A Crisis Within the Pandemic. Indian J Otolaryngol Head Neck Surg 75, 523–528 (2023). https://doi.org/10.1007/s12070-022-03318-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03318-4