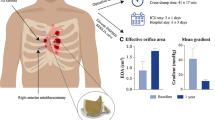
Abstract
Purpose
This study aims to evaluate the perioperative outcomes of aortic valve replacement (AVR) via right anterior minithoracotomy (RAT) during the learning curve.
Methods
It was a retrospective, observational, cohort study of patients who underwent RAT AVR from June 2015 to April 2022. Primary outcomes measured were 30-day morbidity and mortality.
Results
A total of 107 consecutive patients underwent elective RAT AVR. Our patients were mostly male (78.5%), elderly (mean 68.7 years), and obese (34.6%). A majority of the patients (93.5%) were of low operative risk. Median cross-clamp and bypass times were 95 and 123 minutes respectively. There was a statistically significant correlation between increase in number of cases and decrease in operative time. All patients had no paravalvular leak at discharge. There were no operative cardiovascular mortality or major morbidity including stroke, myocardial infarction, renal failure requiring dialysis, or vascular complication. No patient required intraoperative conversion to full sternotomy for completion of AVR.
Conclusion
Our study demonstrated that RAT AVR can be safely introduced. The learning curve required in performing RAT AVR can be safely negotiated through training, previous experience in minimally invasive surgery, careful patient selection including use of preoperative computed tomography of the aorta, and introduction of sutureless/rapid deployment valves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sternal-sparing aortic valve replacement (AVR) has evolved in the last few decades and the conduct of AVR is now possible through a limited (5–7 cm) right-sided incision. However, AVR through right anterior minithoracotomy (RAT) is still not widespread due to the associated technical challenges and the required steep learning curve. This is the largest Australian study to date, evaluating the outcomes of RAT AVR during the learning curve.
Materials and methods
Patient selection and data collection
This was a retrospective, observational, cohort study of prospectively collected data from 107 consecutive patients who underwent RAT AVR at four institutions by a single surgeon from June 2015 to April 2022. Data was collected from patient records, anesthetic and perfusion charts. Outcomes measured were operative morbidity and mortality within 30 days of the operation.
Preoperative planning and surgical technique
Patients with significant atheroma in the aortic arch or descending thoracic aorta based on preoperative computed tomography (CT) were excluded due to the risk of retrograde embolization in the initial experience, when femoral artery was routinely used for inflow cannulation. Subsequently when direct aortic cannulation was routinely performed, only those with substantial ascending aortic atheroma were excluded from the RAT procedure. Patients were also considered unsuitable if they had substantial pectus, or if they required concomitant coronary arterial bypass grafting or significant aortic procedure.
RAT technique was performed with a 5-cm incision in the second intercostal space from the lateral border of the sternum (Fig. 1). The third sternocostal cartilage was divided, and the right internal mammary artery and vein were ligated. Aortic cannulation was achieved peripherally through the transfemoral approach. With increasing experience, central aortic cannulation was performed primarily for subsequent cases, while still maintaining excellent operative field (Fig. 2). Venous cannulation was established percutaneously with Seldinger technique under transesophageal echocardiographic (TOE) guidance through the right common femoral vein using a multi-staged venous cannula.
Intraoperative views through a RAT incision. The focussed view via RAT incision still provided good exposure of pertinent mediastinal structures including the AV (i and ii) and allowed surgical removal of the pathological valve with subsequent thorough surgical debridement of any calcification present; and a full view of the closed aortotomy incision (iv) to check for hemostasis, demonstrating the safety of RAT AVR. Even when aortic cannulation was established centrally (ii and iii), it did not obstruct operative view
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics (Herrenberg, Germany). Continuous variables were expressed as mean ± standard deviation for normally distributed data and median (interquartile ranges) for non-normal data. Categoric data was presented with actual values and percentage of the total. Analysis of the surgeon’s level of experience was completed as a categorical variable with patients chronologically assigned to groups of tens. Differences in operative times were compared using 1 factorial analysis of variance. Statistical significance was defined as a p value of less than 0.05.
Results
From June 2015 to April 2022, a total of 107 patients underwent RAT AVR.
Baseline characteristics
The clinical characteristics of the study population are detailed in Table 1. Our patient population was relatively elderly (mean age 68.7 ± 9.6 years; 65–80 years 65.4%; and > 80 years 7.5%). About a third of our patients (34.6%) had a body mass index (BMI) of more than 30 kg/m2. Most of our cohort (93.5%) were of low operative risk.
Operative data
Surgical details are summarized in Table 2. Arterial cannulation was established peripherally through the common femoral artery for 27 patients (25.2%) and centrally through the ascending aorta for 80 patients (74.8%). Patients were observed to have either tricuspid (49.5%) or bicuspid (47.7%) valve. Unicuspid valve was uncommon (2.8%). Most patients received tissue valve implantation (87.9%).
Twelve patients (11.2%) had concomitant procedures at the time of RAT AVR as detailed in Table 2. Eleven patients (10.3%) had significant calcification requiring extensive decalcification of aortic root, left ventricular outflow tract, interventricular septum, aortomitral curtain, and/or anterior mitral leaflet, with 1 patient requiring reconstruction of aortomitral curtain and base of anterior mitral leaflet using pericardial patch. One patient had an aortic root enlargement.
Operative time according to surgeon’s experience is illustrated in Fig. 3. As demonstrated, there was a statistically significant decline in both cross-clamp time (134 minutes in initial period vs 90 minutes in latest period, p = 0.02) and cardiopulmonary bypass time (178 minutes in initial period vs 124 minutes in latest period, p = 0.01) with increasing operative experience. Overall, median cross-clamp and cardiopulmonary bypass time were 95 and 123 minutes respectively. One patient (0.9%) required second cross-clamping to repair mild paravalvular leak. All patients had good aortic valvular function with no paravalvular leak confirmed on intraoperative TOE after weaning off bypass and on predischarge echocardiogram.
Clinical outcomes
Clinical outcomes at 30 days are shown in Fig. 4. Perioperatively, there were no cases of cardiovascular mortality or stroke. There was 1 non-cardiac death. This patient died from respiratory failure after being readmitted for acute exacerbation of pre-existing interstitial lung disease.
None of the patients required conversion to full sternotomy intraoperatively for completion of RAT AVR. Four patients (3.7%) required reoperation for postoperative bleeding, of which 2 cases had sternotomy while the other 2 patients had reoperation via the same thoracotomy incision in later part of our series. Twelve patients (11.2%) required red blood cell transfusion. Drain output at 4 hours was 130 (75–200) ml. At the time of discharge, mean hemoglobin level was 111.7 ± 16.8 g/L.
Atrial fibrillation occurred in 38 (35.5%) patients. Thirteen (12.1%) patients required permanent pacemaker (PPM) implantation, of which 6 patients had balloon expandable sutureless or rapid deployment valves. For patients who required PPM implantation, 2 patients had complete heart block while 11 patients had tachy-brady syndrome and received PPM for safe administration of rate-controlling medications. At post-discharge follow up, device check was again performed on these patients which confirmed that only 2 patients had persistent complete heart block requiring PPM.
Three patients (2.8%) required prolonged ventilation and 1 (0.9%) patient required reintubation for respiratory failure due to obesity and pre-existing obstructive sleep apnea. One patient (0.9%) had infective endocarditis requiring reoperation 24 days after the initial surgery. As shown in Fig. 4, there were no cases of myocardial infarction, need for mechanical circulatory support, acute renal injury requiring dialysis, or vascular complication.
Mean duration of intensive care unit (ICU) stay was 2 days (2.4 ± 1.7 days). Of note, more than half of our patients were operated in private facilities where patients were routinely observed in ICU for at least 48 hours. Mean duration of hospital stay was 8 days (7.6 ± 3.4 days).
Discussion
Despite its advantages, RAT AVR is still not widely adopted. This may largely be attributed to the required additional training and the associated steep learning curve. Our study, which is the largest Australian report of its kind, has demonstrated that RAT AVR may be safely introduced as a routine procedure while getting over the learning curve.
While rates of mortality and stroke for full sternotomy AVRs have previously been reported to be between 1.4–1.9% and 1.2–1.3% respectively on national databases [1, 2], there were no cases of cardiovascular mortality or stroke in our patients. The zero incidence of perioperative stroke observed in our study was likely the result of careful patient selection (which involved the use of preoperative gated CT aortogram) and intraoperative conduct of central aortic cannulation in majority of our patients. Hence, we suggest the routine use of CT aortogram for planning of RAT AVR to minimize the occurrence of perioperative stroke.
The avoidance of sternotomy in RAT AVR has eliminated sternal-related complications, reduced pain and decreased loss of blood while providing better cosmesis, and increased patient satisfaction. Although deep sternal wound infections are uncommon in isolated full-sternotomy AVR, with a reported incidence of only 0.7% in Australasia [1], they are still associated with poorer outcomes including increased mortality [3]. With the sternal-sparing approach in RAT AVR, the risk of sternal infection was fully eradicated with no cases of postoperative wound infection observed in our patients, which is particularly beneficial in patients with elevated BMI. Despite having a large proportion of patients with a BMI of more than 25 kg/m2, none of our patients required intraoperative conversion to full sternotomy for completion of AVR. In addition, it is possible to perform RAT AVR without division of the third sternocostal junction but this may limit exposure and necessitate femoral arterial cannulation, adding to the risk of perioperative stroke. In our experience, the division of costal cartilages did not result in any major adversity or significant pain issues. There were no formal measurements of postoperative pain or mobility in our study. However, we observed that our patients experienced lesser pain as compared to other minithoracotomy approaches in our unit. Also, we notice a reduced requirement for red blood cell transfusion in our patients, which was half of the reported binational average for isolated AVR (11.2% vs 25.8%) [1]. This may be due to the complete preservation of sternum in RAT AVR. Mean hemoglobin level at time of discharge was more than 100 g/L, indicating minimal blood loss during or post-RAT surgery.
Operative time was longer in our series as observed in other minimally invasive surgeries. Nevertheless, it is important to note that this has not translated into adverse clinical outcomes. In addition, operative time in our study has reduced considerably while getting over the learning curve. This was mainly attributed to training and cumulative experience with performing RAT AVR over the years. Other factors which helped overcome the learning curve safely included previous experience in other minimally invasive surgeries, careful patient selection and preoperative planning involving the use of CT aortogram, as well as the introduction of rapid deployment and sutureless valve technology to decrease operation time.
With continued advancements in medical technology, transcatheter aortic valve implantation (TAVI) has emerged as a potential alternative to surgical AVR [4] and its application has now been extended to the low surgical risk group [5, 6]. The emergence of TAVI has led to an increased interest in sternal-sparing approaches but there are no large randomized trials directly comparing the outcomes of RAT AVR with TAVI. Some ongoing concerns with TAVI use include its risk of paravalvular leak, its limited application in bicuspid aortic stenosis, and the lack of data to demonstrate its long-term durability [5, 6]. We believe that a well-established RAT AVR program is an ideal alternative to percutaneous valves, especially for young patients with low operative risks or patients who are unsuitable candidates for TAVI.
While performing a successful RAT AVR is demonstrated to be achievable in our study, having it widely adopted remains challenging due to institutional and surgeon-related factors. Institutions need to be willing to invest in training and in purchasing new equipment, while surgeons have to maintain an open mind to perform minimally invasive AVR despite the already well-performed low-risk full sternotomy AVR. It is possible to develop technical skills to get over the learning curve of RAT AVR without compromising patient outcomes.
Limitations of study
We acknowledge the retrospective nature of this study, which has its limitations adherent to its study design. Also, this study has evaluated only a single surgeon’s technique and outcomes. Therefore, the outcomes here may not necessarily apply for every other surgeon. However, the technique used is well-defined and so should be reproducible.
Conclusions
In summary, our study has demonstrated that RAT AVR may be safely introduced as a routine procedure after getting over the learning curve. It is possible to overcome the learning curve safely with training, careful patient selection and preoperative planning, cumulative operative experience with RAT AVR, and use of rapid deployment or sutureless aortic valves. Although performing RAT AVR involves relatively long operative time and requires a steep learning curve, it provides good cosmesis, preserving sternal integrity with no significant impact on major clinical outcomes.
References
Shardey G, Tran L, Williams-Spence J, Solman N, McLaren J, Marrow N, et al. The Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database Program National Annual Report 2020. Monash Uni, DEPM, 2021; Report No 14.
Bowdish ME, D’Agostino RS, Thourani VH, Schwann TA, Krohn C, Desai N, et al. The Society of Thoracic Surgeons adult cardiac surgery database: update on outcomes and research. Ann Thorac Surg. 2021;111:1770–80.
Kaspersen AE, Nielsen SJ, Orrason AW, Petursdottir A, Sigurdsson MI, Jeppsson A, et al. Short- and long-term mortality after deep sternal wound infection following cardiac surgery: experience from SWEDEHEART. Eur J Cardiothorac Surg. 2021;60:233.
Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Sebagh L, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43:698–703.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705.
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expandable valve in low-risk patients. N Engl J Med. 2019;380:1706–15.
Acknowledgements
We would like to thank Dr Marc Sakwa MD, for his guidance prior to the establishment of RAT AVR program in Perth.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics committee approval
Approval from local ethics committee was obtained for the conduct of this study.
Informed consent
Written patient consent has been obtained for photographs to be used for publication.
Human and animal rights statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chia, R.H.(., Joshi, P. Sternal sparing aortic valve replacement via right anterior minithoracotomy: An early experience. Indian J Thorac Cardiovasc Surg 40, 191–197 (2024). https://doi.org/10.1007/s12055-023-01596-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-023-01596-3