Abstract
Purpose
The Norwood procedure, the first surgical step of staged palliation for hypoplastic left heart syndrome (HLHS), is also applied for other complex single ventricle lesions. This study aimed to evaluate the outcome of the Norwood operation in a single center over 4 years and to identify clinical and anatomic risk factors for overall mortality.
Methods
A retrospective review of the pediatric cardiovascular surgery database was performed to identify infants with HLHS who underwent NP (Norwood procedure) at our institution between January 2007 and December 2011. Our study population consisted of 85 patients with HLHS.
Results
Early mortality (30 days postoperative period) between January 2007 and December 2011 for Norwood operation was 7 (8.2%) out of 85 patient, and overall mortality was 24 (28.2%).
Conclusion
Our single-center experience shows that the Norwood operation can be performed for complex single ventricle lesions with similarly good early outcomes regardless of the underlying anatomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Four decades ago, treatment options for hypoplastic left heart syndrome (HLHS) were limited owing to high mortality rates [1, 2]. Therefore, in most centers, patients were advised, either to terminate pregnancy or provide compassionate care after birth. HLHS and other related single right ventricle malformations are among the most serious form of congenital heart defects, accounting for about 25% of cardiac mortality in the first week of life in the absence of surgical intervention [3].
The outcome of patients with HLHS has dramatically improved because of the refinement of surgical techniques and perioperative care [4]. Tweddell et al. reported that between July 1996 to October 2001, hospital survival was about 93% (75/81) compared with that of 53% (18/34) from January 1992 to June 1996 [5]. McGuirk et al. published articles estimating that early mortality declined from 100% (95% CI 21 to 100%) in December 1992 to 17% in September 1995. Later data suggested that early mortality was 10% in June 2004 [6].
The goals of the Norwood operation are (1) to provide unobstructed systemic blood flow through a reconstructed neoaortic arch, (2) to control pulmonary blood flow so that the pulmonary arterial bed will develop normally, and (3) to create an unrestricted communication between the atria via an atrial septectomy [7, 8].
Compared with the right ventricle-to-pulmonary artery (RV-PA) shunt, higher interstage mortality has been reported in the modified Blalock–Taussig shunt (MBTS) [9, 10]. RV-PA type of Norwood procedure might have lower interstage mortality as there is no diastolic runoff with a potential coronary steal [11, 12].
We reported our institutional experience with the Norwood procedure as first-stage palliation for HLHS and noted a remarkable reduction of early mortality within the study period. The aims of our study were to analyze the outcomes of patients with HLHS and to improve understanding of the risk factors associated with overall mortality following RV-PA shunt palliation of HLHS.
Methods
A retrospective review of the pediatric cardiovascular surgery database was performed to identify infants with HLHS who underwent NP (Norwood procedure) at our institution between January 2007 and December 2011. Our study population consisted of 85 patients with HLHS including those who required atrial septal or other catheter-based interventions in the pre-Norwood period. We reviewed data on anatomy, pre, peri, and postoperative details, and risk factors for overall mortality. Anatomic data were obtained on the basis of echocardiography performed before the Norwood operation. Preoperatively, all children were treated according to a standardized regimen consisting of strict afterload reduction with sodium nitroprusside, furosemide, enteral feeding, and avoidance of mechanical ventilation or inotropic support.
Inclusion criteria consisted of a diagnosis of HLHS or a related single ventricle anomaly and a planned Norwood procedure. Exclusion criteria were the preoperative identification of cardiac anatomy that would render the RV-PA shunt technically impossible, any major congenital defect or acquired extracardiac abnormality that could independently affect the likelihood of transplantation-free survival and patients with severe neurological disorders.
Operative technique
A conventional median sternotomy is performed, and the thymus gland is partially dissected, thus exposing the aortic arch and branch vessels, and preserved. To maintain hemodynamic stability, minimal dissection is carried out prior to institution of cardiopulmonary bypass (CPB). The infant is cannulated with a single venous cannula through the right atrial appendage, and a systemic perfusion cannula is inserted through the mid portion of the ductus arteriosus. CPB is instituted with snaring of the ductus arteriosus, and the infant is cooled to 18 °C. During cooling, the branch vessels of the aortic arch are exposed and looped with silicone elastomer snares in preparation for circulatory arrest. The ascending, transverse, and distal portions of the aortic arch are completely dissected, and the proximal ductus is divided and oversewn. The proximal anastomosis site of the RV-PA conduit was determined within the infundibulum in relation to coronary vessels. Crystalloid cardioplegia was infused through the aortic cannula after the occlusion of pulmonary arteries, head vessels, and descending aorta. The “septum primum” was resected after removing the atrial cannula through the atrial appendage. The main pulmonary artery was divided before the bifurcation. After the excision of all remnants of the ductal tissue and the elongation of the incision far beyond the coarctation (when present), the descending, transverse, and ascending aortas were reconstructed with a pulmonary artery homograft. The proximal pulmonary artery was then anastomosed to the reconstructed neoaorta. The CPB was reinstituted. A right ventriculostomy was made with a 5.0-mm aortic punch under the pulmonary annulus, and the 5-mm RV-PA conduit was placed on the left or the right side of the neoaorta. After the construction of distal connection of the homograft cuff to the distal part of the main pulmonary artery, proximal direct anastomosis of the tube graft to the muscle of the infundibulum of the right ventricle with a continuous (5.0) suture was done during rewarming.
Pulmonary homograft were used in all of our norwood patients. Pulmonary perfusion was achieved with a RV-PA conduit (Sano shunt), and hemofiltration was used routinely.
Postoperatively, all patients were continued on afterload reduction therapy with sodium nitroprusside. For anticoagulation, 3 mg/kg acetylsalicylic acid was routinely given. Patients were discharged from the hospital when they could be fed orally without a gastric tube, were thriving, and had an oxygen saturation greater than 75%. Outcomes for risk factors after the Norwood operation were obtained by statistical analysis. Death after the Norwood operation, regardless of whether in or out of the hospital, was defined as overall mortality.
Statistical methods
Descriptive statistics presented include median with interquartile range for skewed variables, mean, and standard deviation for other continuous variables and the frequency with percentage for categorical variables.
Univariate logistic regression and logistic regression adjusted for site were used to obtain initial estimates of the association between overall mortality and each candidate predictor. Predictors significant at P less than 15 at the univariate or site-adjusted level were included in the multivariable modeling. After the multivariable model main effects were determined, the significant interactions were added and retained if significant at a P value less than 15.
Results
Patient characteristics in the study are presented in Table 1. There were 26 (30.6%) female and 59 (69.4%) male patients. Prenatal diagnosis was made in 25 (29.4%) patients. Mean weight of patients was 3244 ± 548.11 g. The number of aortic arch reconstructions using homograft patches was 72 (84.7%), and the number of pulmonary trunk sewn into the aortic arch for aortic arch construction was 7 (8.4%). Average saturation at the time of discharge was 74.54 ± 8.9%.
Univariate analysis of risk factors for overall mortality is shown in Table 2. Risk factors included mitral atresia, restriction of atrial septum, CPB pump stops, duration of CPB, average saturation at the time of discharge, and coarctation of the aorta. Multivariate analyses of risk factors for overall mortality are shown in Table 3. The most significant risk factors were the duration of CPB and restriction of the atrial septum.
Discussion
Four decades ago, the prognosis for infants born with HLHS was considered uniformly fatal, as 95% did not survive the first month of life. HLHS was the most common cause of death due to heart disease in the newborn period [13]. As a result of the pioneering efforts over the past decade by Norwood et al. [14] in the area of palliative surgery, and by Bailey et al. [15], Backer et al. [16], and their associates in the area of cardiac transplantation, the outlook for these infants has dramatically improved.
This article explores the outcomes of NP for HLHS in our institution. The vast majority of these patients had the extreme form of HLHS with aortic atresia, restrictive atrial septum, coarctation of the aorta, and 2 mm or less diameter of ascending aorta with aortic arch construction in 79 patients.
Despite the prevalence of these anatomic characteristics, 30 days postoperative mortality between January 2007 and December 2011 for Norwood operation at our institution was 7 out of 85 (8.2%) patients, and overall mortality was 24 (28.2%). A notable decrease in early mortality after the Norwood procedure has also been reported by other groups [5]. The reasons for this achievement are certainly numerous—an overall gain of experience during the study period, decrease in the duration of the cardiac ischemia time, and deep hypothermic circulatory arrest (DHCA) time with the follow-up interval. Sufficient afterload reduction has also certainly substantially contributed [17]. It has been shown by Tweddell et al. [5] that a sufficient afterload reduction helps to avoid an abrupt increase in systemic vascular resistance in the postoperative course and, in conjunction with manipulating inflammatory response, accounts for the reduction in early mortality.
The effects of timing of NP on patient outcomes have been the focus of prior investigations, although the evidence in the literature is mixed. Although older age at time of NP was initially thought to be associated with better outcomes [18], other data have demonstrated either worse outcomes [19] or no difference in outcomes after NP [20]. Infants may undergo NP safely at an age of ≥ 7 days [21]. Subjects who had an MBTS were at higher risk for interstage mortality than those with RV-PA shunt. With pulmonary blood flow occurring only during systole after palliation with the RV-PA shunt, a higher diastolic pressure and lower pulmonary to systemic flow ratio have been observed [22, 23]. It is possible that the higher diastolic pressure associated with RV-PA shunt contributes to improved coronary and systemic perfusion, providing advantageous hemodynamic stability during periods of stress such as illness or feeding difficulties. Patients with an MBTS may have a higher risk of acute shunt thrombosis; however, MBTS was not examined in this analysis. Jonas et al. [24] found the combination of aortic atresia with mitral stenosis to be associated with interstage mortality for infants with HLHS. Our single-center investigation found mitral atresia to be a risk factor. Other investigators reviewing single-center series have not found this anatomic variant to be a risk factor [25]. These data suggest that risk for overall mortality in RV-PA shunt is not a consequence of postoperative regurgitation of the tricuspid valves. However, due to smaller size of the study, we cannot conclusively demonstrate that moderate to severe regurgitation of tricuspid valves is a risk factor, possibly secondary to the additional volume load and ineffective ventricular output that can be exacerbated by conditions that elevate systemic vascular resistance.
We found that restrictive atrial septal defects and coarctation of the aorta were independent risk factors for interstage mortality. Risk factors such as intact atrial septum and coarctation of the aorta are stronger determinants of post-Norwood mortality as suggested by our data.
Other studies have identified extracardiac anomalies, birth weight, associated cardiac anomalies, total support time, and extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD) support as predictors of operative mortality [26, 27]. The increased risk of overall mortality with longer total support time was confirmed by our study. Longer length of stay was not an independent risk factor for interstage mortality. Longer length of stay in other series of infants with serious congenital heart disease has been associated with negative outcomes, including early mortality and lower scores on neurodevelopmental testing [28].
Poor interstage growth and malnutrition are common after the NP [29]; thus, alternative feeding methods may be necessary to ensure adequate nutritional support. However, the best approach to feeding children after the NP remains a challenge and there is a wide variation in practice.
Home monitoring with daily oxygen saturations, weight measurements and with increased communication, has been associated with reduced interstage mortality in some series [30, 31] and may be of particular value when access to pediatric cardiac care is not readily available. Its impact on overall mortality was not evaluated in this analysis.
The construction of the neoaorta can be more complex with HLHS. Different techniques including transactions of both vessels, pulmonary artery, and ascending aorta or techniques using two patches to avoid a single spiral-shaped patch have been described [32,33,34]. We prefer a single patch to avoid multiple anastomotic sites.
In our study, we assessed the impact of risk factors on overall mortality. Regurgitation of tricuspid valve, an ascending aortic diameter of 2 mm or less, and low weight at operation were not found to increase the risk of overall mortality after Norwood operation. As procedural risk factors, the use of DHCA and longer duration of total support time were associated with a higher risk for overall mortality.
The main finding of our study was that the Norwood operation can be applied with good early results regardless of the underlying anatomy and ventricular morphology.
Limitations
Our current study is limited by its single-center, retrospective design of a relatively small number of patients, although all available data were used in all statistical analyses.
Conclusions
Our single-center experience shows that the Norwood operation can be performed for complex single ventricle lesions with good early outcomes regardless of the underlying anatomy. These results are encouraging for the treatment of single ventricle lesions with a systemic right ventricle.
Discussant:
Dr. SMRUTI RANJAN MOHANTY Consultant Pediatric Cardiac Surgeon KOKILABEN DHIRUBHAI AMBANI HOSPITAL FOUR BUNGLOWS, ANDHERI(W) MUMBAI, INDIA
Congratulations for the outstanding results in these difficult substrates of patients in the experience you have reported. For the benefit of the readers I wish to take you through few questions whose answers in your context might add to our understanding of the anomaly.
Q1 You had an exclusion criteria of few kids where the anatomy did not allow a RV to PA shunt. Can you elaborate the substrates a bit more and the reasons why the Sano shunt could not be feasible? Assuming that your group believes in the RV-PA shunt in stage I palliation, optimal option (MBTS/RV-PA/HYBRID) which is divided among the major centers offering Norwood, do you think it was possible to offer the MBTShunt option in those kids who according to you could not qualify for a RV-PA shunt?
A1 Exclusion criteria included such coronary anatomy, which make ventriculotomy technically difficult. Yes it is possible to offer MBTS shunt option in these kids.
Q2 You have mentioned a stringent pre operative protocol which follows the standard principles, but I am a bit curious to know the reason behind your selection of SNP in both pre and post operative phase in the context that now advanced dilators as milrinone and levosimendan are available?
A2 Each patient was monitored using routine standard intensive care practices for our pediatric cardiac intensive care unit. Generally in pre - operative period, patients are under care of neonatologist or pediatric cardiologist in their respective departments and are sent to us a few hours prior to surgery. During preoperative periods, babies with HLHS require prostaglandin E to maintain ductal patency. HlLHS with restrictive ASD also require measures aimed at improving pulmonary flow, such as aggressive mechanical ventilation with high inspired oxygen fractions or nitric oxide. They also require interventional cardiac catheterisation for creation or enlargement of a restrictive or absent ASD. For respiratory management there is need for continuous monitoring of their oxygen saturation. The balance between pulmonary flow (Qp) and systemic flow (Qs) is critically important: an excess of one will by definition compromise the other. Hypoxic gas (14–20% FiO2) can be delivered by adding nitrogen to ventilator or ambient gases. Sodium nitroprusside is a vasodilator with a rapid onset of action, and has lot of potential advantages and a short half life. Phenoxybenzamine, an α-adrenergic receptor blocker, was shown to reduce Qp:Qs. Inotropes are not specifically indicated in the preoperative setting, but may be beneficial in some individuals. A low dose infusion of an inotrope such as dobutamine (5–10 μg/kg/min) may improve ventricular function.
Post-operative period : After the Norwood operation, an early echo should be performed to assess the adequacy of the repair. Patients are managed on Morphine infusion and neuromuscular blockers if unstable. Initial inodilator therapy is milrinone (0.5 to 0.7 mcg/kg/min) and low dose adrenaline (0.02 to 0.05 mcg/kg/min). If milrinone is not effective, levosimendan is used. For weaning from bypass, dopamine (3–5 μg/kg/min) and milrinone (0.3–0.8 μg/kg/min) were used. Infusion of these drugs was continued in the PCICU (Paediatric Cardiac Intensive Care Unit) and sodium nitroprusside (1–3 μg/kg/min) and/or epinephrine (0.02–0.05 μg/kg/min) was added when indicated. All patients were given infusion of heparin (5 U/kg/h) during first several days after the NP and replaced with acetylsalicylic acid (2–3 mg/kg/day) during the interstage period.
Q3 In terms of procedure, have you ever considered perfusing through a 3/ 3.5 mm PTFE graft sewn to the innominate artery to continue ACP through out the procedure and thus avoid DHCA which is supposed to be an incremental risk factor in both cardiac and neurological outcomes?
A3 We prefer DHCA to perform Norwood procedure. However few surgeons in our center considered perfusing through a 3/ 3.5 mm PTFE graft sewn to the innominate artery to continue ACP along with DHCA.
Q4 Have you considered using NIRS as a tool to monitor low cardiac out put and cerebral perfusion status in intra op and post op phase?
A4 In our center ,we are using traditional method of deep hypothermic circulatory arrest for modified Norwood procedure. We are not using NIRS in intra op and post op phase.
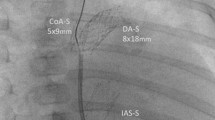
Q5. It was not clear that in the process of reconstructing the Neo Aorta, do you anastomose the end of the splayed opened ascending Aorta to the upper medial edge of the transected proximal MPA with few interrupted sutures (DKS anastomosis), or you believe in the augmentation of the whole Ascending Aorta, Arch and proximal descending Aorta with a pulmonary homograft patch and then make counter incision in this patch corresponding to the MPA proximal stump and then anastomose that in end to side fashion? Please clarify, if possible with a suitable cartoon/illustration.
A5. After the excision of all remnants of the ductal tissue and the elongation of the incision far beyond the coarctation (when present), the descending, transverse and ascending aortas were reconstructed with a pulmonary artery homograft. The proximal pulmonary artery was then anastomosed to the reconstructed neo-aorta. When the junction of the ascending aorta and pulmonary root is reached, the excess patch is trimmed and the proximal connection between the patch and the pulmonary root is completed. After the proximal patch is trimmed, the neoaorta is completed.

Q6. What has been your approach to the coarctation segment and ductal tissue as many of us believe in complete excision of both, mobilization of descending aorta, creation of the neo posterior wall by suturing the splayed upon arch end to the posterior wall of descending aorta and then augmenting the anterior aspect with a homograft patch/ bovine pericardium?
A6. Incision is made uptill distal end of the coarctation segment and ductal tissue is completely excised.
Q7. Have you considered using the DUNK technique for at least the proximal end of the Sano shunt? This has of late improved the graft patency and reduced the re intervention rate, if one goes by the literature.
A7. Yes, Construction of the RV-PA conduit. A ringed Gore-Tex tube graft is brought up and one end is cut flush with the external ring. A marking pen is used to mark the third ring from the end. This ensures that only a millimeter or two of the graft protrudes into the RV cavity.
Q8. It would have been prudent to mention few things in the post op strategy like the number of times chest kept open, and if so for how many days? It would be also nice to have information regarding criteria for extubation/ ICU stay and other major issues in the post op phase like NEC/LCOS etc.
A8. The chest is routinely left splinted open for first 3–5 day post Norwood. At time of chest closure, we are cautious of desaturation from compression of shunt. May need to reopen chest. Not fed enterally for first 3–5 days until chest closed and stable haemodynamics on low doses of inotropes. Early use of TPN. Agents most commonly used include high dose milrinone (0.5 to 1.0 mcg/kg/min). As there is invariably a degree of impaired myocardial contractility post Norwood stage 1, we routinely add low dose epinephrine 0.02 to 0.04 mcg /kg/min to the milrinone infusion. We try not to use high doses of epinephrine, if possible, because of the increased myocardial oxygen consumption and risk. Gently increase FiO2 (titrate up slowly) as precipitous increase in FiO2 can cause a sudden fall in PVR and loss of cardiac output from high pulmonary run off and inadequate coronary perfusion. When all these conventional manoeuvres fail, consider inhaled nitric oxide (an unusual, but not un heard of clinical situation).
Q9. There is no clarity on the issue of tricuspid regurgitation in these kids which I believe is a nagging problem. What was your strategy to deal with this issue?
A9. Initial therapy is milrinone and low dose adrenaline. Often converted to captopril (particularly if any tricuspid regurgitation) after patient extubated and stable. A tricuspid valvuloplasty is now performed more readily at the stage 2 operation whenever regurgitation of that valve is more than mild.
Q10. Did your group believe in elective feeding gastrotomy in any of your kids or you discharged home with nasogastric tube for feeds?
A10. No
Q11. What was the situation in the inter stage and did any kid require any intervention/ readmission before Stage 2? What is your policy of progressing to the stage 2?
A11 Preoperative cardiac catheterisation is routinely required between stage 1 and 2 and 2 and 3 of Norwood operation. Postoperative care after the Norwood 1 operation has now shifted to improving cardiac output and away from the past obsession of balancing Qp:Qs.
The best monitor of adequacy of cardiac output or systemic oxygen delivery is poor perfusion, acidosis, lactic acidosis, poor urine output, high filling pressures, and often poor function on echo and often with low SaO2.
Q12. Lastly did you notice any difference in the group that was prenately diagnosed versus the babies where the diagnosis was done after receiving in your unit after birth?
A12. Prenatal diagnosis can allow for more careful planning of delivery and immediate postnatal care of infants with HLHS. Infants diagnosed prenatally are certainly less likely to experience the sequelae of pulmonary over-circulation or acidosis. For the minority with a restrictive ASD, prenatal diagnosis allows for immediate postnatal intervention.
The prenatal diagnosis can have a negative impact on the fate of the baby, if parents decide to terminate pregnancy. Fetal interventions in HLHS have been developed in order to improve haemodynamics and prevent the development of defects. Currently, two types of treatments are used in this defect: Balloon septostomy in case of significant communication restrictions, and Atrial balloon valvotomy in cases of critical stenosis. These interventions contribute to increased blood flow through “Left heart” and prevent its improper development structurally. Interventions are performed under ultrasound (Fetal echocardiography).
References
Gutgesell HP, Massaro TA. Management of hypoplastic left heart syndrome in a consortium of university hospitals. Am J Cardiol. 1995;76:809–11.
Meliones JN, Snider AR, Bove EL, Rosenthal A, Rosen DA. Longitudinal results after first-stage palliation for hypoplastic left heart syndrome. Circulation. 1990;82:IV151–6.
Watson DG, Rowe RD. Aortic-valve atresia: report of 43 cases. JAMA. 1962;179:14–8.
Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ III. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102:III136–41.
Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–9.
McGuirk SP, Griselli M, Stumper OF, et al. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart. 2006;92:364–70.
Azakie T, Merklinger SL, McCrindle BW, et al. Evolving strategies and improving outcomes of the modified Norwood procedure: a 10-year single-institution experience. Ann Thorac Surg. 2001;72:1349–53.
Weldner PW, Myers JL, Gleason MM, et al. The Norwood operation and subsequent Fontan operation in infants with complex congenital heart disease. J Thorac Cardiovasc Surg. 1995;109:654–62.
Cua CL, Thiagarajan RR, Gauvreau K, et al. Early postoperative outcomes in a series of infants with hypoplastic left heart syndrome undergoing stage 1 palliation operation with either modified Blalock-Taussig shunt or right ventricle to pulmonary artery conduit. Pediatr Crit Care Med. 2006;7:238–44.
Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single ventricle lesions. N Engl J Med. 2010;362:1980–92.
Pizarro C, Mroczek T, Malec E, Norwood WI. Right ventricle to pulmonary artery conduit reduces interim mortality after stage 1 Norwood for hypoplastic left heart syndrome. Ann Thorac Surg. 2004;78:1959–63.
Cua CL, Thiagarajan RR, Taeed R, et al. Improved interstage mortality with the modified Norwood procedure: a meta-analysis. Ann Thorac Surg. 2005;80:44–9.
Fyler DC. Report of the New England regional infant cardiac program. Pediatrics. 1980;65:463.
Norwood WI, Kirklin JK, Sanders SP. Hypoplastie left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45:87–91.
Bailey LL, Assaad AN, Trimm RF, et al. Orthotopic transplantation during early infancy as therapy for incurable congenital heart disease. Ann Thorac Surg. 1988;208:279–86.
Backer CL, Zales VR, Harrison HL, Idriss FS, Benson DW Jr, Mavroudis C. Intermediate-term results of infant orthotopic cardiac transplantation from two centers. J Thorac Cardiovasc Surg. 1991;101:826–32.
Furck AK, Uebing A, Hansen JH. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12-year single-center survey. J Thorac Cardiovasc Surg. 2010;139:359–65.
Rossi AF, Sommer RJ, Steinberg LG, et al. Effect of older age on outcome for stage one palliation of hypoplastic left heart syndrome. Am J Cardiol. 1996;77:319–21.
Alsoufi B, Manlhiot C, Al-Ahmadi M, et al. Older children at the time of the Norwood operation have ongoing mortality vulnerability that continues after cavopulmonary connection. J Thorac Cardiovasc Surg. 2011;142:142–7.
Ghanayem NS, Allen KR, Tabbutt S, et al. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906.
Shamszad P, Gospin TA, Hong BJ, McKenzie ED, Petit CJ. Impact of preoperative risk factors on outcomes after Norwood palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;147:897–901.
Bradley SM, Simsic JM, McQuinn TC, Habib DM, Shirali GS, Atz AM. Hemodynamic status after the Norwood procedure: a comparison of right ventricle-to-pulmonary artery connection versus modified Blalock-Taussig shunt. Ann Thorac Surg. 2004;78:933–41.
Ghanayem NS, Jaquiss RD, Cava JR, Frommelt PC, Mussatto KA, Hoffman GM, et al. Right ventricle-to-pulmonary artery conduit versus Blalock-Taussig shunt: a hemodynamic comparison. Ann Thorac Surg. 2006;82:1603–10.
Jonas RA, Hansen DD, Cook N, Wessel D. Anatomic subtype and survival after reconstructive operation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1994;107:1121–8.
Polimenakos AC, Sathanandam SK, Hussayni TS, El Zein CF, Roberson DA, Ilbawi MN. Hypoplastic left heart syndrome and aortic atresia-mitral stenosis variant: role of myocardial protection strategy and impact of ventriculo-coronary connections after stage 1 palliation. Pediatr Cardiol. 2011;32:929–39.
Gaynor JW, Mahle WT, Cohen MI, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22:82–9.
Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:412–7.
Newburger JW, Wypij D, Bellinger DC, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143:67–73.
Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–44.
Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–77.
Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young. 2006;16:61–6.
Thistlewaite PA, Myers JL, Siewers RD, Ettedgui JA. Technique for repair of single-ventricle hearts with transposition of the great arteries and aortic arch hypoplasia. Ann Thorac Surg. 1999;67:260–2.
Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22:82–9.
Jacobs JP, O’Brien SM, Chai PJ, Morell VO, Lindberg HL, Quintessenza JA. Management of 239 patients with hypoplastic left heart syndrome and related malformations from 1993 to 2007. Ann Thorac Surg. 2008;85:1691–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
In retrospective studies “For this type of study, formal consent is not required.” According to the Polish law, approval from the Ethics Committee was not required for retrospective data analysis, since this kind of study did not violate human rights. All parents of those children provided written informed consents for surgery.
Rights and permissions
About this article
Cite this article
Rai, V., Mroczek, T., Szypulski, A. et al. Outcome of Norwood operation for hypoplastic left heart syndrome. Indian J Thorac Cardiovasc Surg 34, 337–344 (2018). https://doi.org/10.1007/s12055-017-0603-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-017-0603-1