Abstract
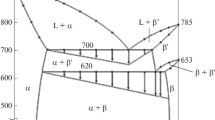
A theoretical model based on compound formation in binary alloys has been used to investigate the energetics of liquid Ag–Cu, Ag–Sb and Cu–Sb binaries at 1423, 1250 and 1190 K, respectively. The Gibbs free energies of mixing, component thermodynamic activities, enthalpies and entropies of mixing were fitted with available experimental data to determine the energetics of the liquid mixture. Investigations were done to understand the nature of chemical order in the bulk of the systems and to predict the nature of the alloys’ surfaces. Good agreements between the theoretical model and available experimental data were accompanied by satisfactory and reliable predictions of surface properties in the binary systems. This work showed that amongst Ag, Cu and Sb atoms that form the three binaries, tendency of forming heterocoordinated bonds is the highest for Sb atoms, and the studies carried out on the surfaces of Ag–Cu, Ag–Sb and Cu–Sb binaries reveal that the surface of Ag–Cu–Sb ternary will be Sb-enriched and Cu-deficient in most compositions. This work has improved the understanding of the thermodynamics of Ag–Cu, Ag–Sb and Cu–Sb binaries, as well as contributed knowledge for a better understanding of the Ag–Cu–Sb ternary.








Similar content being viewed by others
References
G K Gupta and A Dixit, Int. J. Energy Res. 44, 3724 (2020)
J Li, Y Li, Z Wang, H Bian, Y Hou, F Wang, G Xu, B Liu and Y Liu, Sci. Rep. 6, 1 (2016)
M Premović, D Minić, D Manasijević, D Živković and J Djokić, J. Alloys Compd. 548, 249 (2013)
M Aspiala and P Taskinen, J. Chem. Thermodyn. 93, 261 (2016)
D Gurešić, A Dordević, A Marković, M Tomović, N Talijan, and I Manasijević, J. Eng. Process. Manag. 8, 45 (2016)
A A Ajayi and E D Ogunmola, Int. J. Adv. Mater. Res. 4, 29 (2018)
M Gautam, Asian J. Adv. Res. 5, 48 (2020)
A Krzyzak and K Fitzner, Thermochim. Acta 414, 115 (2004)
N Panthi, I B Bhandari and I Koirala, Himal. J. Sci. Technol. 3, 68 (2020)
J Brillo, E Arato, D Giuranno, H Kobatake, C Maran, R Novakovic, E Ricci and D Rosello, High Temp. Press. 47, 417 (2018)
I S Jha, I Koirala, B P Singh and D Adhikari, Appl. Phys. A Mater. Sci. Process. 116, 1517 (2014)
A B Bhatia and R N Singh, Phys. Chem. Liq. 11, 343 (1982)
B C Anusionwu and O K Echendu, Phys. Chem. Liq. 48, 127 (2020)
J Brillo, G Lauletta, L Vaianella, E Arato, D Giuranno, R Novakovic and E Ricci, ISIJ Int. 54, 2115 (2014)
J P Ansermet and S D Brechet, Principles of thermodynamics (Cambridge University Press, Cambridge, 2019)
Y A Odusote and A Popoola, Niger. J. Pure Appl. Phys. 9, 11 (2020)
A B Bhatia and R N Singh, Phys. Chem. Liq. 13, 177 (1984)
A B Bhatia and W H Hargrove, Phys. Rev. B 10, 3186 (1974)
O M Oshakuade and O E Awe, Phys. Sci. Rev. 8, 3 (2023)
O M Oshakuade and O E Awe, Submitted for publication. n.d. arXiv:2102.08516 [cond-mat.mtrl-sci]
Y Waseda, The structure of non-crystalline materials: Liquids and amorphous solids (McGraw-Hill, New York, 1980)
R N Singh, Can. J. Phys. 65, 309 (1987)
U Khair, H Fahmi, S Al Hakim and R Rahim, J. Phys.: Conf. Ser. 930, 012002 (2017)
M E Trybula, P W Szafrański and P A Korzhavyi, J. Mater. Sci. 53, 8285 (2018)
O E Awe and O Olawole, J. Non. Cryst. Solids 358, 1491 (2012)
H K Limbu, K K Mishra, G P Adhikari and I S Jha, Int. J. Theor. Math. Phys. 10, 33 (2020)
R P Koirala, S K Yadav, B P Singh, I S Jha and D Adhikari, Himal. Phys. 6, 15 (2017)
L C Prasad, R N Singh and G P Singh, Phys. Chem. Liq. 273, 179 (1994)
R Hultgren, P D Desai, D T Hawkins, M Geiser and K K Kelley (Eds), Selected values of the thermodynamic properties of binary alloys (ASM, Metals Park, OH, 1973)
A A Ajayi, E D Ogunmola and A E Adeoye, Am. J. Condens. Matter Phys. 7, 67 (2017)
L C Prasad, R N Singh, V N Singh and S K Chatterjee, Phys. B Phys. Condens. Matter 215, 225 (1995)
V I Berdnikov and Y A Gudim, Izv. Ferr. Metall. 60, 241 (2017)
R Novakovic, E Ricci, D Giuranno and A Passerone, Surf. Sci. 576, 175 (2005)
Y Tang, C Huang, Z Yang, Y Wang, M Huang, F Huang, J Tu and Z Zhou, Philos. Mag. 100, 2128 (2020)
T Iida and R I L Guthrie, The physical properties of liquid metals (Clarendon Press, Oxford, 1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajayi, A.A., Oyeniyi, E. & Oshakuade, O.M. Bulk and surface properties of liquid Ag–Cu, Ag–Sb and Cu–Sb alloys. Pramana - J Phys 97, 72 (2023). https://doi.org/10.1007/s12043-023-02536-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12043-023-02536-x