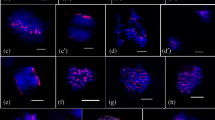
Histones are the major eukaryotic DNA-binding proteins. Posttranslational modifications on N-terminal tails of histones that form nucleosomes are often associated with distinct biological functions. Some theories suggest that one of these modifications, the phosphorylation of histone H3 at serine 10 (H3S10ph) plays a role in both chromosome condensation and sister chromatid cohesion. Although histones and some of their modifications are highly conserved, studies have shown that role and distribution of H3S10ph may differ between species. We evaluated the pattern of H3 phosphorylation using immunodetection during mitosis and meiosis in both diploid and tetraploid genotypes of Brachiaria species. Results revealed differences in chromosome distribution of H3S10ph when mitosis and meiosis were compared. Whole chromosomes were phosphorylated during meiosis I, whereas phosphorylation was restricted to the pericentromeric region in both meiosis II and mitosis. There was no variation in phosphorylation patterns between Brachiaria species and diploid and tetraploid genotypes. Regarding spatiotemporal coordination in the Brachiaria species evaluated, H3S10ph is related to maintenance of sister chromatid cohesion during cell divisions.






Similar content being viewed by others
References
Bannister A. J. and Kouzarides T. 2011 Regulation of chromatin by histone modifications. Cell Res. 21, 381–395.
Bardan A. 2010 Many functions of the meiotic cohesion. Chromosome Res. 18, 909–924.
Chen Z. J. 2007 Genetic and epigenetic mechanisms for gene expression an phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 58, 377–406.
Chen M., Shaolei L. V. and Meng Y. 2010 Epigenetic performers in plants. Dev. Growth Differ. 52, 555–566.
Eckert C. A., Gravdahl D. J. and Megee P. C. 2007 The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev. 21, 278–291.
Feitoza L. and Guerra M. 2011 Different types of plant chromatin associated with modified histones H3 and H4 and methylated DNA. Genetica 139, 305–314.
Fernandes T., Yuyama P. M., Moraes A. P. and Vanzela A. L. 2008 An uncommon H3/Ser10 phosphorylation pattern in Cestrum strigilatum (Solanaceae), a species with B chromosomes. Genome 51, 772–777.
Germand D., Demidov D. and Houben A. 2003 The temporal and spatial pattern of histone H3 phosphorylation at serine 28 and serine 10 is similar in plants but differs between mono- and polycentric chromosomes. Cytogenet. Genome Res. 101, 172–176.
Guerra M., Brasileiro-Vidal A. C., Arana P. and Puertas M. J. 2006 Mitotic microtubule development and histone H3 phosphorylation in the holocentric chromosomes of Rhynchospora tenuis (Cyperaceae). Genetica 41, 126–133.
Hans F. and Dimitrov S. 2001 Histone H3 phosphorylation and cell division. Oncogene 20, 3021–3027.
Hendzel M. J., Wei Y. and Mancini M. A. 1997 Mitosis specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360.
Hirano T. 2002 The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 16, 399–414.
Houben A., Wako T., Shimogawara R. F., Presting G., Künzel G., Schubert I. and Fukui K. 1999 The cell cycle dependent phosphorylation of histone H3 is correlated with the condensation of plant mitotic chromosomes. Plant J. 18, 675–679.
Houben A., Demidov D., Caperta A. D., Karimi R., Agueci F. and Vlasenko L. 2007 Phosphorylation of histone H3 in plants: a dynamic affair. Biochim. Biophy. Acta 1769, 308–315.
Jin W., Lamb J. C., Zhang W., Kolano B., Birchler J. A. and Jiang J. 2008 Histone modifications associated with both A and B chromosomes of maize. Chromosome Res. 16, 1203–1214.
Johansen K. M. and Johansen J. 2006 Regulation of chromatin structure by histone H3S10 phosphorylation. Chromosome Res. 14, 393–404.
Kaszás E. and Cande W. Z. 2000 Phosphorylation of histone H3 is correlated with changes in the maintenance of sister chromatid cohesion during meiosis in maize, rather than the condensation of the chromatin. J. Cell Sci. 113, 3217–3226.
Kouzarides T. 2007 Chromatin modifications and their function. Cell 128, 693–705.
Li Z. H., Lu X., Gao Y., Liu S., Tao M., Xiao H. et al. 2011 Polyploidization and epigenetics. Chin. Sci. Bull. 56, 245–252.
Losada A., Hirano M. and Hirano T. 1998 Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 12, 1986–1997.
Manzanero S., Arana P., Puertas M. J. and Houben A. 2000 The chromosomal distribution of phosphorylated histone H3 differs between plants and animals at meiosis. Chromosoma 109, 308–317.
Nasmyth K. and Haering C. H. 2009 Cohesin: its roles and mechanisms. Annu. Rev. Genet. 43, 525–558.
Qiao H., Lohmiller L. D. and Anderson L. K. 2011 Cohesin proteins load sequentially during prophase I in tomato primary microsporocytes. Chromosome Res. 19, 193–207.
Schroeder-Reiter E., Houben A. and Wanner G. 2003 Immunogold labelling of chromosomes for scanning electron microscopic: a closer look at phosphorylated histone H3 in mitotic metaphase chromosomes of Hordeum vulgare. Chromosome Res. 11, 585–596.
Ünal E., Arbel-Eden A., Sattler U., Shroff R., Lichten M., Haber J. E and Koshland D. 2004 DNA damage response pathway uses histone modification to assemble a doublestrand break-specific cohesin domain. Mol. Cell 16, 991–1002.
Wei Y., Mizzen C. A., Cook R. G., Gorovsky M. A. and Allis C. D. 1998 Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc. Natl. Acad. Sci. USA 95, 7480–7484.
Zhang X., Li X., Marchall J. B., Zhong C. X. and Dawe R. K. 2005 Phosphoserines on maize Centromeric Histone H3 and Histone H3 Demarcate the Centromere and Pericentromere during chromosome segregation. Plant Cell 17, 572–583.
Acknowledgements
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support to research, and M. S. Ludmila Cristina Oliveira for contribution in preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Paula C. M. P., Techio V. H., Sobrinho F. S. and Freitas A. S. 2013 Distribution pattern of histone H3 phosphorylation at serine 10 during mitosis and meiosis in Brachiaria species. J. Genet. 92, xx–xx]
Rights and permissions
About this article
Cite this article
PAULA, C.M.P., TECHIO, V.H., SOBRINHO, F.S. et al. Distribution pattern of histone H3 phosphorylation at serine 10 during mitosis and meiosis in Brachiaria species. J Genet 92, 259–266 (2013). https://doi.org/10.1007/s12041-013-0261-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-013-0261-z