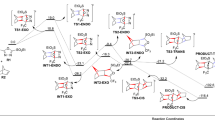
Abstract
Spherical conformational landscape model was revisited to include yet another class of cyclic compounds; the derivatives of cyclohexane. The updated model is not only capable of explaining Raman spectral features in fluxional cyclopentane but is also capable of revealing similarities between cyclopentane and cyclohexane derivatives for the first time. At the heart of the model lies the aspect of B/T ring coordinates (B/T conformational platform) that represents different levels of puckering (q). DFT-ωb97xd/6-311 + G* computations confirmed by MP2/aug cc-pVTZ computations were used to fully investigate 16 different derivatives of both cyclohexane and cyclopentane. Intrinsic reaction coordinate, IRC, computations were performed to gain insight into patterned inter-platform pathways connecting ring coordinates. These pathways revealed the coupling strength between bent/boat, B, and twist, T, ring coordinates. The coupling is found to be stronger for cyclopentane compared to cyclohexane. Some spectral features in the overlap region near 1400 cm−1 show promising signs on spin–spin relaxation, T2, mechanism. The work opens up an avenue for conformational studies of medium-sized rings. Also, ongoing studies to unravel potential relationships between conformational flexibility and bioactivity of cyclic compounds are underway.
Graphic abstract
Conformationally mobile cyclic carbonic skeletons like cis-Octahydropentalenes are occurring in a number of natural products. A complete conformational analysis of cis-Octahydropentalenes reveals how some natural products populate their bioactive form. The pattern successfully explains the bioactive conformation in (+)-epi-goniofufrone and Hirsutic acid, both of which are natural products with potent bioactivity. Analysis like these are illustrative of how nature can use some special scaffolds to achieve maximal bioactivity, which in turn would fuel research in drug design.






Similar content being viewed by others
References
Allinger N L 1964 Conformational analysis in the elementary organic course J. Chem. Edu. 41 70
Strauss H L and Pickett H M 1970 Conformational structure, energy, and inversion rates of cyclohexane and some related oxanes J. Am. Chem. Soc. 92 7281
Leonard J E, Hammond G S and Simmons H E 1975 Apparent symmetry of cyclohexane J. Am. Chem. Soc. 97 5052
Leventis N, Hanna S B and Sotiriou-Leventis C 1997 A three-dimensional energy surface for the conformational inversion of cyclohexane J. Chem. Edu. 74 813
Nelson D J and Brammer C N 2010 Toward consistent terminology for cyclohexane conformers in introductory organic chemistry J. Chem. Edu. 88 292
Kakhiani K, Lourderaj U, Hu W, Birney D and Hase W L 2009 Cyclohexane Isomerization. Unimolecular Dynamics of the Twist-Boat Intermediate J. Phys. Chem. A 113 4570
Brügger G, Frey H-M, Steinegger P, Balmer F and Leutwyler S 2011 Accurate determination of the structure of cyclohexane by femtosecond rotational coherence spectroscopy and ab initio calculations J. Phys. Chem. A 115 9567
McGrath K J and Weiss R G 1993 Rate of chair-to-chair interconversion of cyclohexane-d12 in its neat plastic crystalline phase J. Phys. Chem. 97 2497
Dasgupta S, Tang Y, Moldowan J M, Carlson R M and Goddard III W A 1995 Stabilizing the boat conformation of cyclohexane rings J. Am. Chem. Soc. 117 6532
Wiberg K B, Castejon H, Bailey W F and Ochterski J 2000 Conformational studies in the cyclohexane series. 2. Phenylcyclohexane and 1-methyl-1-phenylcyclohexane J. Org. Chem. 65 1181
Wiberg K B, Hammer J D, Castejon H, Bailey W F, DeLeon E L and Jarret R M 1999 Conformational studies in the cyclohexane series. 1. Experimental and computational investigation of methyl, ethyl, isopropyl, and tert-butylcyclohexanes J. Org. Chem. 64 2085
Lipnick R L 1974 NMR spectroscopy of cyclopentane derivatives. III. Methylcyclopentane J. Am. Chem. Soc. 96 2941
Poupko R, Luz Z and Zimmermann H 1982 Pseudorotation in cyclopentane. An experimental determination of the puckering amplitude by NMR in oriented solvents J. Am. Chem. Soc. 104 5307
Pitzer K S and Donath W E 1983 Conformations and strain energy of cyclopentane and its derivatives. In: Molecular Structure and Statistical Thermodynamics: Selected Papers of Kenneth S Pitzer (World Scientific) pp. 98–103
Tomimoto M and Go N 1995 Analytic theory of pseudorotation in five-membered rings. Cyclopentane, tetrahydrofuran, ribose, and deoxyribose J. Phys. Chem. 99 563
Ocola E J, Bauman L E and Laane J 2011 Vibrational spectra and structure of cyclopentane and its isotopomers J. Phys. Chem. A 115 6531
Kowalewski P, Frey H-M, Infanger D and Leutwyler S 2015 Probing the structure, pseudorotation, and radial vibrations of cyclopentane by femtosecond rotational Raman coherence spectroscopy J. Phys. Chem. A 119 11215
Sakhaee N, Jalili S and Darvish F 2016 Spherical conformational landscape shed new lights on fluxional nature of cyclopentane and its derivatives, confirmed by their Raman spectra Comput. Theor. Chem. 1090 193
Ross B D and True N S 1983 NMR spectroscopy of cyclohexane. Gas-phase conformational kinetics J. Am. Chem. Soc. 105 4871
Offenbach J L, Fredin L and Strauss H L 1981 Vibrational spectra of twist-boat cyclohexane J. Am. Chem. Soc. 103 1001
Halgren T A 1996 Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions J. Comput. Chem. 17 520
Cao H-Y, Si D-H, Tang Q, Zheng X-F and Hao C 2016 Electronic structures and solvent effects of unsymmetrical neo-confused porphyrin: DFT and TDDFT–IEFPCM investigations Comput. Theor. Chem. 1081 18
Goerigk L and Grimme S 2010 Efficient and Accurate Double-Hybrid-Meta-GGA Density Functionals—Evaluation with the Extended GMTKN30 Database for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions J. Chem. Theory Comput. 7 291
Shao Y, Molnar L F, Jung Y, Kussmann J, Ochsenfeld C, Brown S T, Gilbert A T, Slipchenko L V, Levchenko S V and O’Neill D P 2006 Advances in methods and algorithms in a modern quantum chemistry program package Phys. Chem. Chem. Phys. 8 3172
Hratchian H P, Parandekar P V, Raghavachari K, Frisch M J and Vreven T 2008 QM: QM electronic embedding using Mulliken atomic charges: Energies and analytic gradients in an ONIOM framework J. Chem. Phys. 128 034107
Frisch M, Trucks G, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B and Petersson G (2009) Gaussian 09, revision a 02, Gaussian. Inc., Wallingford, CT 2009 200
Tang Y, Shan X, Niu S, Liu Z, Wang E, Watanabe N, Yamazaki M, Takahashi M and Chen X 2017 Electron momentum spectroscopy investigation of molecular conformations of ethanol considering vibrational effects J. Phys. Chem. A 121 277
Dong W, Yang H, Qiu Z and Tang Y 2019 Electron momentum spectroscopy of cyclopentane: A molecular dynamical investigation J. Electron Spectro. Relat. Phenom. 230 40
Aksnes D W, Førland K and Stöcker M 2005 1H NMR relaxation and diffusion studies of cyclohexane and cyclopentane confined in MCM-41 Micropor. Mesopor. Mater. 77 79
Aliev A E and Harris K D 1997 Dynamic Properties of Cyclohexane Guest Molecules Constrained within the Zeolite H-ZSM-5 Host Structure: A Wide-Line Solid State 2H NMR Investigation J. Phys. Chem. A 101 4541
Huang Y and Leech J H 2003 An FT-Raman spectroscopic study of the conformational properties of chlorocyclohexane in zeolites J. Phys. Chem. B 107 7647
Quach J Q 2013 Disorder-correlation-frequency-controlled diffusion in the Jaynes-Cummings–Hubbard model Phys. Rev. A 88 053843
Dehghani A, Mojaveri B, Shirin S and Faseghandis S A 2016 Parity Deformed Jaynes-Cummings Model: “Robust Maximally Entangled States” Sci. Rep. 6 38069
Wu A, Cremer D, Auer A A and Gauss J 2002 Extension of the karplus relationship for NMR spin − spin coupling constants to nonplanar ring systems: pseudorotation of cyclopentane J. Phys. Chem. A 106 657
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors would like to declare no conflict of interest in this work as presented.
Rights and permissions
About this article
Cite this article
SAKHAEE, S., SAKHAEE, M.H., TAKALLOU, A. et al. Fluxional nature in cyclohexane and cyclopentane: spherical conformational landscape model revisited. J Chem Sci 132, 11 (2020). https://doi.org/10.1007/s12039-019-1701-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1701-y