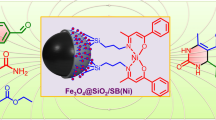
Abstract
Nanomagnetic \(\hbox {Fe}_{{3}} \hbox {O}_{{4}} @ \hbox {SiO}_{{2}}\hbox {-SO}_{{3}}\hbox {H}\) (\(\hbox {SO}_{{3}}\hbox {H-MNPs}\)) was prepared via grafting sulfonic acid on the silica-coated \(\hbox {Fe}_{{3}} \hbox {O}_{{4}}\) magnetite nanoparticles (MNPs). The catalytic activity of the prepared \(\hbox {SO}_{{3}}\hbox {H-MNPs}\) was probed through the one-pot synthesis of N-hydroxy-\({\upalpha }\)-amino phosphonates and \({\upalpha }\)-amino phosphonates via three-component couplings of phenylhydroxylamine or amines with aldehydes and trialkyl phosphites at room temperature. The synthesized \(\hbox {SO}_{{3}}\hbox {H-MNPs}\) were characterized by XRD, FT-IR, and SEM. The recoverability of the catalyst was achieved by a simple magnetic decantation and reused at least five times without significant degradation in catalytic activity.
Graphical Abstract
The catalytic activity of the prepared \(\hbox {SO}_{{3}}\hbox {H-MNPs}\) was probed through the one-pot synthesis of N-hydroxy-\({\upalpha }\)-amino phosphonates and \({\upalpha }\)-amino phosphonates via three-component couplings of phenylhydroxylamine or amines with aldehydes and trialkyl phosphites at room temperature.








Similar content being viewed by others
References
Ramakrishna K, Thomas J M and Sivasankar C 2016 A Green Approach to the Synthesis of \(\alpha \)-Amino Phosphonate in Water Medium: Carbene Insertion into the N-H Bond by Cu(I) Catalyst J. Org. Chem. 81 9826
Allen M C, Fuhrer W, Tuck B, Wade R and Wood J M 1989 Renin inhibitors. Synthesis of transition-state analog inhibitors containing phosphorus acid derivatives at the scissile bond J. Med. Chem. 32 1652
Atherton F R, Hassal C H and Lambert R W 1986 Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)phosphonic acid J. Med. Chem. 29 29
Meyer J H and Barlett P A 1998 Macrocyclic Inhibitors of Penicillopepsin. 1. Design, Synthesis, and Evaluation of an Inhibitor Bridged between P1 and P3 J. Am. Chem. Soc. 120 4600
Miller D J, Hammond S M, Anderluzzi D and Bugg T D H 1998 Aminoalkylphosphinate inhibitors of D-Ala-D-Ala adding enzyme J. Chem. Soc. Perkin Trans. 1 131
Peyman A, Stahl W, Wagner K, Ruppert D and Ruppert K H X 1994 Non-peptide-based inhibitors of human immune deficiency virus-1 protease Bioorg. Med. Chem. Lett. 4 2601
Ali S A N, Ali S, Zakir S, Patel M and Farooqui M 2012 Synthesis of new \(\alpha \)-aminophosphonate system bearing Indazole moiety and their biological activity Eur. J. Med. Chem. 50 39
Maier L and Diel P J 1991 Organic phosphorus compounds 97. Synthesis and properties of 1-amino-2-aryl- and 2-pyridylethylphosphonic acids and derivatives Phosphorus, Sulfur Silicon Relat. Elem. 62 15
Sobhani S and Khakzad F 2017 A novel hydrophobic copper complex supported on \(\gamma \)-Fe2O3 as a magnetically heterogeneous catalyst for one-pot three-component synthesis of \(\alpha \)-aminophosphonates Appl. Organometal. Chem. 31 e3877
Nakamura Y and Ukita T 2002 Construction of Heterocyclic Compounds by Use of \(\alpha \)-Diazophosphonates:? New One-Pot Syntheses of Indoles and Isocoumarins Org. Lett. 14 2317
Debrouwer W, Heugebaert T S A and Stevens C V 2014 Preparation of Tetrasubstituted 3-Phosphonopyrroles through Hydroamination: Scope and Limitations J. Org. Chem. 10 4322
Cheng X, Goddard R, Buth G and List B 2008 Direct Catalytic Asymmetric Three-Component Kabachnik–Fields Reaction Angew. Chem. Int. Ed. 47 5079
Zefirov N S and Matveeva E D 2008 Catalytic Kabachnik-Fields reaction: New horizons for old reaction Arkivoc part 1 1
Cherkasov R A and Galkin V I 1998 The Kabachnik–Fields reaction: Synthetic potential and the problem of the mechanism Russ. Chem. Rev. 67 857
Kafarski P, Gorniak MGV and Andrasiak I 2015 Kabachink-Fields reaction under green conditions – A critical overview Curr. Green. Chem. 2 218
Keglevich G and Balint E 2012 The Kabachnik-Fields Reaction: Mechanism and Synthetic Use Molecules 17 12821
Bera K, Nadkarni D and Namboothiri I N N 2013 Asymmetric synthesis of \(\gamma \gamma \)-aminophosphonates: The Bio-isosteric analogs of \(\gamma \gamma \)-aminobutyric acid J. Chem. Sci. 125 443
Qian C and Huang T 1998 One-Pot Synthesis of \(\alpha \)-Amino Phosphonates from Aldehydes Using Lanthanide Triflate as a Catalyst J. Org. Chem. 63 4125
Tang J, Wang T, Zhang L, Wu S and Mao D 2011 A facile synthesis of \(\alpha \)-aminophosphonates catalyzed by ytterbium perfluorooctanoate under solvent-free conditions J. Fluorine Chem. 132 102
Paraskar A S and Sudalai A 2006 A novel \({\text{ Cu(OTf) }}_{2}\) mediated three component high yield synthesis of \(\alpha \)-aminophosphonates Arkivok 2006 183
Ranu B C, Hajra A and Jana U 1999 General Procedure for the Synthesis of \(\alpha \)-Amino Phosphonates from Aldehydes and Ketones Using Indium(III) Chloride as a Catalyst Org. Lett. 1 1141
Xu F, Luo Y, Deng M and Shen Q 2003 One-Pot Synthesis of \(\alpha \)-Amino Phosphonates Using Samarium Diiodide as a Catalyst Precursor Eur. J. Org. Chem. 2003 4728
Shamrao T D, Sandip R K, Sandeep S K, Thandankorai G S and Radha V J 2012 Choline chloride.\(\text{2ZnCl }_{{2}}\) ionic liquid: An efficient and reusable catalyst for the solvent free Kabachnik–Fields reaction Tetrahedron Lett. 53 2277
Laschat S and Kunz H 1992 Carbohydrates as Chiral Templates: Stereoselective Synthesis of (R)- and (S)-\(\alpha \) Aminophosphonic Acid Derivatives Synthesis 1992 90
Chandrasekhar S, Prakash S J, Jagadeshwar V and Narsihmulu C 2001 Three component coupling catalyzed by \(\text{ TaCl }_{{5}}-\text{ SiO }_{{2}}\): Synthesis of \(\alpha \)-amino phosphonates Tetrahedron Lett. 42 5561
Sivala M R, Devineni S R, Golla M, Ametla V M, Pothuru G K and Chamarthi N R 2016 A heterogeneous catalyst, \(\text{ SiO }_{{2}}\)-\(\text{ ZnBr }_{{2}}\): An efficient neat access for \(\alpha \)-aminophosphonates and antimic robial activity evaluation J. Chem. Sci. 128 1303
Kaboudin B and Nazari R 2001 Microwave-assisted synthesis of 1-aminoalkyl phosphonates under solvent-free conditions Tetrahedron Lett. 42 8211
Bhagat S and Chakraborti A K 2007 An Extremely Efficient Three-Component Reaction of Aldehydes/Ketones, Amines, and Phosphites (Kabachnik-Fields Reaction) for the Synthesis of \(\alpha \)-Aminophosphonates Catalyzed by Magnesium Perchlorate J. Org. Chem. 72 1263
Heydari A, Zarei M, Alijanianzadeh R and Tavakol H 2001 One-pot synthesis of N-trimethylsilyloxy-\(\alpha \)-amino phosphonates from aldehydes using lithium perchlorate/diethyl ether as a catalyst Tetrahedron Lett. 42 3629
Gallardo-Macias R and Nakayama K 2010 Tin(II) Compounds as Catalysts for the Kabachnik-Fields Reaction under Solvent-Free Conditions: Facile Synthesis of \(\alpha \)-Aminophosphonates Synthesis 1 57
Akiyama T, Sanada M and Fuchibe K 2003 Brønsted Acid-Mediated Synthesis of \(\alpha \)-Amino Phosphonates under Solvent-Free Conditions Synlett 10 1463
Manabe K and Kobayashi S 2000 Facile synthesis of \(\alpha \)-aminophosphonates in water using a Lewis acid–surfactant-combined catalyst Chem. Commun. 0 669
Ha H J and Nam G S 1992 Expedient Synthesis of \(\alpha \)-Substituted \(\alpha \), \(\beta \)- Unsaturated \(\gamma \)-Amino Acids (Dipeptide Mimetics); Wittig Reaction of \(\alpha \)-Amino Aldehydes with \(\alpha \)- Substituted Alkoxycarbonyl Phosphoranes Synth. Commun. 22 1143
Mohammadian E, Ghafuri H and Kakanejadifard A 2017 A new procedure for synthesis of \(\alpha \)-aminophosphonates by aqueous formic acid as an effective and environment-friendly organocatalyst J. Chem. Sci. 129 1883
Vahdat S M, Baharfar R, Tajbakhsh M, Heydari A, Baghbanian S M and Khaksar S 2008 Organocatalytic synthesis of \(\alpha \)-hydroxy and \(\alpha \)-aminophosphonates Tetrahedron Lett. 49 6501
Dindulkar S D, Reddy M V and Jeong V T 2012 Cd \((\text{ ClO }_{4})_{2} \cdot \text{ xH }_{{2}}\text{ O }\) as a novel catalyst for the synthesis of \(\alpha \)-aminophosphonates under solvent-free conditions Catal. Commun. 17 114
Reddy C B, Kumar K S, Kumar M A, Reddy M V N, Krishna B S, Naveen M, Arunasree M K, Reddy C S, Raju C N and Reddy C D E 2012 PEG-\(\text{ SO }_{{3}}\text{ H }\) catalyzed synthesis and cytotoxicity of \(\alpha \)-aminophosphonates Eur. J. Med. Chem. 47 553
Joshi R S, Mandhane P G, Dabhade S K and Gill C H 2010 Potassium dihydrogen phosphate: An inexpensive reagent for the solvent-free, One-pot synthesis of \(\alpha \)-aminophosphonates Green Chem. Lett. Rev. 3 191
Hamadi H, Kooti M, Afshari M, Ghiasifar Z and Adibpour N 2013 Magnetic nanoparticle supported polyoxometalate: An efficient and reusable catalyst for solvent-free synthesis of \(\alpha \)-aminophosphonates J. Mol. Catal. A: Chem. 373 25
AhmadDar B, Chakraborty A, Sharma P R, Shrivastava V, Bhowmik A, Vyas D, Bhatti P, Sharma M and Singh B 2013 Grinding-induced rapid, Convenient and solvent free approach for the one pot synthesis of \(\alpha \)-aminophosphonates using aluminium pillared interlayered clay catalyst J. Ind. Eng. Chem. 19 732
Malamiri F and Khaksar S 2014 Pentafluorophenylammonium triflate (PFPAT): A new organocatalyst for the one-pot three-component synthesis of \(\alpha \)-aminophosphonates J. Chem. Sci. 126 807
Kumar K S, Krishna B S, Reddy C B, Reddy M V N and Reddy C S 2017 Solvent-free synthesis of \(\alpha \)-aminophosphonates: Cellulose-\(\text{ SO }_{{3}}\text{ H }\) as an efficient catalyst Arab. J. Chem. 10 S368
Goulioukina N S, Shergold I A, Bondarenko G N, Ilyin M M, Davankov V A and Beletskaya I P 2012 Palladium-Catalyzed Asymmetric Hydrogenation of N-Hydroxy-\(\alpha \)-imino Phosphonates Using Brønsted Acid as Activator: The First Catalytic Enantioselective Approach to Chiral N-Hydroxy-\(\alpha \)-amino Phosphonates Adv. Synth. Catal. 345 2727
Gouverneure V and Lalloz M H 1996 N-Hydroxy-\(\alpha \)-amino phosphonate derivatives as potential haptens for eliciting catalytic antibodies Tetrahedron Lett. 37 6331
Hubber R and Vasella A 1990 The kinetic anomeric effect. Additions of nucleophiles and of dipolarophiles to N-glycosylnitrones and to N-pseudoglycosylnitrones Tetrahedron 46 33
De Risi C, Dondoni A, Perroneb D and Pollinia G P 2001 O-Silyl triflate-promoted addition of diethyl phosphite to chiral aldonitrones. A rapid access to complex \(\alpha \)-amino phosphonates and their N-hydroxy derivatives Tetrahedron Lett. 42 3033
Yadav J S, Reddy B V S and Sreedhar P 2003 Three-Component One-Pot Synthesis of \(\alpha \)-Hydroxylamino Phosphonates Using Ionic Liquids Adv. Synth. Catal. 34 564
Lim C W and Lee I S 2010 Magnetically recyclable nanocatalyst systems for the organic reactions Nano Today 5 412
Shylesh S, Schunemann V and Thiel W R 2010 Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis Angew. Chem. Int. Ed. 49 3428
Riente P, Mendoza C and Pericas M A 2011 Functionalization of \(\text{ Fe }_{{3}} \text{ O }_{{4}}\) magnetic nanoparticlesfor organocatalytic Michael reactions J. Mater. Chem. 21 7350
Abu-Rezig R, Alper H, Wang D and Post M L 2006 Metal Supported on Dendronized Magnetic Nanoparticles:? Highly Selective Hydroformylation Catalysts J. Am. Chem. Soc. 128 5279
Kiasat A R and Davarpanah J 2013 \(\text{ Fe }_{{3}} \text{ O }_{{4}}\)@silica sulfuric acid nanoparticles: An efficient reusable nanomagnetic catalyst as potent solid acid for one-pot solvent-free synthesis of indazolo[2,1-b]phthalazine-triones and pyrazolo[1,2-b]phthalazine-diones J. Mol. Catal. A: Chem. 373 46
Kiasat A R and Davarpanah 2015 \(\text{ Fe }_{{3}} \text{ O }_{{4}}\)@Silica sulfuric acid core–shell composite as a novel nanomagnetic solid acid: Synthesis, Characterization and application as an efficient and reusable catalyst for one-pot synthesis of 3,4-dihydropyrimidinones/thiones under solvent-free conditions Res. Chem. Intermed. 41 2991
Ghasemzadeh M A and Azimi-Nasrabad M 2016 Nano-\(\text{ Fe }_{{3}} \text{ O }_{{4}}\) -encapsulated silica particles bearing sulfonic acid groups as a magnetically separable catalyst for the green and efficient synthesis of 14-aryl-14H-dibenzo[a,i]xanthene-8,13-dione derivatives Res. Chem. Intermed. 42 1057
Bhattacharya A K and Rana K C 2008 Amberlite-IR 120 catalyzed three-component synthesis of \(\alpha \)-amino phosphonates in one-pot Tetrahedron Lett. 49 2598
Wu J, Sun W, Xia H G and Sun X 2006 A facile and highly efficient route to \(\alpha \)-amino phosphonates via three-component reactions catalyzed by \(\text{ Mg } (\text{ ClO }_{4})_{2}\) or molecular iodine Org. Biomol. Chem. 4 1663
Yan Y, Li S, Ou Y, Ji Y, Yu Z, Liu L, Yan C, Zhang Y and Zhao Y 2014 Effects of pressure and deposition time on the characteristics of \(\text{ In }_{{2}} \text{ Se }_{{3}}\) films grown by magnetron sputtering Electron. Mater. Lett. 10 1093
Azad S, Sadeghi E, Parvizi R and Mazaheri A 2017 Fast response relative humidity clad-modified multimode optical fiber sensor with hydrothermally dimension controlled ZnO nanorods Mater. Sci. Semicond. Process. 66 200
Ingham B and Toney M F 2014 X-ray diffraction for characterizing metallic films In Metallic Films for Electronic, Optical and Magnetic Applications Katayun Barmak and Kevin Coffey (Eds.) \(1^{{\rm st}}\) edn. (Cambridge: Woodhead Publishing) p. 3
Acknowledgements
The authors thank Shahid Chamran University of Ahvaz for financial support (Grant No. 31400.02.3.95).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamadi, H., Norouzi, M. \({\hbox {SO}}_{3}\hbox {H}\)-functionalized magnetic \({\hbox {Fe}}_{3} {\hbox {O}}_{4}\) nanoparticles as an efficient and reusable catalyst for one-pot synthesis of \({\upalpha }\)-amino phosphonates. J Chem Sci 130, 128 (2018). https://doi.org/10.1007/s12039-018-1530-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1530-4