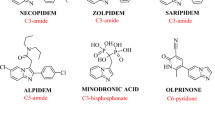
Abstract
The properties of aminophosphonates as transition state analogs of amino acids, and as antibacterial, antifungal and antiHIV agents attracted considerable attention in recent years. Although many reviews appeared in the literature covering α- and β-aminophosphonates, γ-aminophosphonates did not receive sufficient attention despite the fact that parent γ-aminophosphonic acid and its derivatives are bio-isosteric analogs of GABA (γ-amino butyric acid). This review provides a critical summary of the significance of γ-aminophosphonates and various approaches to their synthesis, with particular emphasis to asymmetric versions.

The properties of aminophosphonates as transition state analogs of amino acids, and as anti-bacterial, antifungal and antiHIV agents attracted considerable attention. γ-Aminophosphonic acid in particular is a bio-isosteric analog of GABA (γ-aminobutyric acid).



















































Similar content being viewed by others
References
(a) Kukhar V P and Hudson H R 2000 Aminophosphonic and aminophosphinic acids: Chemistry and biological activity. Chichester: John Wiley; p 634; (b) Kafarski P and Lejczak B 2001 Curr. Med. Chem.: Anti-Cancer Agents 1 301; (c) Ntai I, Manier M L, Hachey D L and Bachmann B O 2005 Org. Lett. 7 2763
Nishikawa M and Kuriyama K 1989 Neurochem. Int. 14 85
Vigot R and Batini C 1999 Neurosci. Res. 34 141
Wiesner J, Henschker D, Hutchinson D B, Beck E and Jomaa H 2002 Antimicrob. Agents Chemother. 46 2889
Ma D 1999 Bioorg. Chem. 27 20
Ordóñez M, Labastida-Galván V and Lagunas-Rivera S 2010 Tetrahedron: Asymmetry 21 129
For reviews on α-aminophosphonates: (a) Lewkowski J 2005 Focus on Organometallic Chem. Res. 167; (b) Syamala M 2005 Org. Prep. Proc. Int. 37 103; (c) Ordóñez M, Rojas-Cabrera H and Cativiela C 2009 Tetrahedron 65 17; (d) Alfonsov V A 2008 Phosphorus, Sulfur, Silicon, Rel. Elem. 183 2637
For reviews on β-aminophosphonates: (a) Palacios F, Alonso C and de los Santos J M 2005 Chem. Rev. 105 899; (b) Enders D, Saint-Dizier A, Lannou M-I and Lenzen A 2005 Eur. J. Org. Chem. 29; (c) Mikołajczyk M 2005 J. Organomet. Chem. 690 2488; (d) Ma J-A 2006 Chem. Soc. Rev. 35 630
Kosolapoff G M 1947 J. Am. Chem. Soc. 69 2112
Chambers J R and Isbell A F 1964 J. Org. Chem. 29 832
Berry J P, Isbell A F and Hunt G E 1972 J. Org. Chem. 37 4396
Brigot D, Collignon N and Savignac P 1979 Tetrahedron 35 1345
Fabre G, Collignon N and Savignac P 1981 Can. J. Chem. 59 2864
Logusch E W 1986 Tetrahedron Lett. 27 5935
Crooks S L, Robinson M B, Koerner J F and Johnson R L 1986 J. Med. Chem. 29 1988
Hall R G 1989 Synthesis 442
Howson W, Hills J M, Blackburn G M and Broekman M 1991 Bioorg. Med. Chem. Lett. 1 501
Kudzin Z H, Kotyñski A and Andrijcwski G 1994 J. Organomet. Chem. 479 199
Kroona H B, Peterson N L, Koerner J F and Johnson R L 1991 J. Med. Chem. 34 1692
Kurdyumova N R, Ragulin V V and Tsvetkov E N 1997 Mendeleev Commun. 7 69
Amori L, Costantino G, Marinozzi M, Pellicciari R, Gasparini F, Flor P J, Kuhn R and Vranesic I 2000 Bioorg. Med. Chem. Lett. 10 1447
Hale J J, Doherty G, Toth L, Li Z, Mills S G, Hajdu R, Keohane C A, Rosenbach M, Milligan J, Shei G J, Chrebet G, Bergstrom J, Card D, Rosen H and Mandala S 2004 Bioorg. Med. Chem. Lett. 14 3495
Johnson R L and Rao K S S P 2005 Bioorg. Med. Chem. Lett. 15 57
Vicario J, Aparicio D and Palacios F 2009 J. Org. Chem. 74 452
Duan Z-C, Hu X-P, Zhang C and Zheng Z 2010 J. Org. Chem. 75 8319
Ma D, Ma Z, Jiang J, Yang Z and Zheng C 1997 Tetrahedron: Asymmetry 8 889
Sibille P, Lopez S, Brabet I, Valenti O, Oueslati N, Gaven F, Goudet C, Bertrand H-O, Neyton J, Marino M J, Amalric M, Pin J-P and Acher F C 2007 J. Med. Chem. 50 3585
Barton D H R and Embse R A V 1998 Tetrahedron 54 12475
Zeiss H-J 1992 Tetrahedron 48 8263
Feng Y and Coward J K 2006 J. Med. Chem. 49 770
Selvam C, Goudet C, Oueslati N, Pin J-P and Acher F C 2007 J. Med. Chem. 50 4656
Xiao Y, Lee K and Liu P 2008 Org. Lett. 10 5521
Tong G, Perich J W and Johns R B 1990 Tetrahedron Lett. 31 3759
Wiemann A, Frank R and Tegge W 2000 Tetrahedron 56 1331
Yokomatsu T, Sato M and Shibuya S 1996 Tetrahedron: Asymmetry 7 2743
De la Cruz A, He A, Thanavaro A, Yan B, Spilling C D and Rath N P 2005 J. Organomet. Chem. 690 2577; (b) Shabany H and Spilling C D 1998 Tetrahedron Lett. 39 1465
Langlois N, Rojas-Rousseau A and Decavallas O 1996 Tetrahedron: Asymmetry 7 1095
Wen X and Hultin P G 2004 Tetrahedron Lett. 45 17735
Foss F W Jr, Snyder A H, Davis M D, Rouse M, Okusa M D, Lynch K R and Macdonald T L 2007 Bioorg. Med. Chem. 15 663
Högenauer K, Hinterding K and Nussbaumer P 2010 Bioorg. Med. Chem. Lett. 20 1485
Otaka A, Miyoshi K, Burke T R Jr, Roller P P, Kubota H, Tamamura H and Fujii N 1995 Tetrahedron Lett. 36 927
Yokomatsu T, Murano T, Akiyama T, Koizumi J, Shibuya S, Tsuji Y, Soeda S and Shimeno H 2003 Bioorg. Med. Chem. Lett. 13 229
Yamagishi T, Muronoi S, Hikishima S, Shimeno H, Soeda S and Yokomatsu T 2009 J. Org. Chem. 74 6350
Natchev I A 1988 Tetrahedron 44 6455
Chakravarty P K, Greenlee W J, Parsons W H, Patchett A A, Combs P, Roth A, Busch R D and Mellin T N 1989 J. Med. Chem. 32 1886
Cordero R D N, Nunez E H, Zertuche M F, Hernandez M M and Ordonez M 2005 Arkivoc 277
Chauvel E N, Llorens-Cortes C, Coric P, Wilk S, Roques B P and Fournie-Zaluski M C 1994 J. Med. Chem. 37 2950
Hale J J, Neway W, Mills S G, Hajdu R, Keohane C A, Rosenbach M, Milligan J, Shei G-J, Chrebet G, Bergstrom J, Card D, Koo G C, Koprak S L, Jackson J J, Rosen H and Mandala S 2004 Bioorg. Med. Chem. Lett. 14 3351
Lu X, Byun H-S and Bittman R 2004 J. Org. Chem. 69 5433
Tarnowski A, Retz O, Bär T and Schmidt R R 2005 Eur. J. Org. Chem. 1129
Sun C and Bittman R 2004 J. Org. Chem. 69 7694
(a) Shie J-J, Fang J-M, Wang S-Y, Tsai K-C, Cheng Y-S E, Yang A-S, Hsiao S-C, Su C-Y and Wong C-H 2007 J. Am. Chem. Soc. 129 11892; (b) Shie J-J, Fang J-M and Wong C-H 2008 Angew. Chem., Int. Ed. 47 5788
Bessis A S, Vadesne G, Bourrat E, Bertho G, Pin J P and Acher F C 2003 Amino Acids 24 303
(a) Robiette R and Marchand-Brynaert J 2001 J. Chem. Soc. Perkin Trans. 2 11 2155; (b) Robiette R, Defacqz N, Stofferis J and Marchand-Brynaert J 2003 Tetrahedron 59 4167
Ruiz-Gómez G, Francesch A, Iglesias M J, López-Ortiz F, Cuevas C and Serrano-Ruiz M 2008 Org. Lett. 10 3981
Villanueva J M, Collignon N, Guy A and Savignac P 1983 Tetrahedron 39 1299
Łyżwa P and Mikołajczyk M 2010 Pure Appl. Chem. 82 577
Minowa N, Hirayama M and Fukatsu S 1984 Tetrahedron Lett. 25 1147
Soloshonok V A, Belokon Y N, Kuzmina N A, Maleev V I, Svistunova N Y, Solodenko V A and Kukhar V P 1992 J. Chem. Soc. Perkin Trans. 1 1525
Castelot-Deliencourt G, Roger E, Pannecoucke X and Quirion J C 2001 Eur. J. Org. Chem. 3031
Schöllkopf U 1983 Pure Appl. Chem. 55 1799
Schick A, Kolter T, Giannis A and Sandhoff K 1995 Tetrahedron 51 11207
Ojea V, Ruiz M, Shapiro G and Pombo-Villar E 1994 Tetrahedron Lett. 35 3273
Ojea V, Ruiz M, Shapiro G and Pombo-Villar E 2000 J. Org. Chem. 65 1984
Ojea V, Fernández M C, Ruiz M and Quintela J M 1996 Tetrahedron Lett. 37 5801
Ruiz M, Fernández M C, Díaz A, Quintela J M and Ojea V 2003 J. Org. Chem. 68 7634
Fernández M C, Quintela J M, Ruiz M and Ojea V 2002 Tetrahedron: Asymmetry 13 233
Fernández M C, Díaz A, Guillín J J, Blanco O, Ruiz M and Ojea V 2006 J. Org. Chem. 71 6958
Fernández M C, Ruiz M, Ojea V and Quintela J M 2002 Tetrahedron Lett. 43 5909
Reyes-Rangel G, Maranon G, Avila-Ortiz C G, de Parrodi C A, Quintero L and Juaristi E 2006 Tetrahedron 62 8404
Amori L, Serpi M, Marinozzi M, Costantino G, Diaz M G, Hermit M B, Thomsen C and Pellicciari R 2006 Bioorg. Med. Chem. Lett. 16 196
Marinozzi M, Serpi M, Amori L, Diaz M G, Costantino G, Meyer U, Flor P J, Gasparini F, Heckendorn R, Kuhn R, Giorgi G, Hermit M B, Thomsen C and Pellicciari R 2007 Bioorg. Med. Chem. 15 3161
Widianti T, Hiraga Y, Kojima S and Abe M 2010 Tetrahedron: Asymmetry 21 1861
Maerten E, Cabrera S, Kjaersgaard A and Jørgensen K A 2007 J. Org. Chem. 72 8893
Zhao D, Wang Y, Mao L and Wang R 2009 Chem. Eur. J. 15 10983
Frydenvang K, Hansen J J, Krogsgaard-Larsen P, Mitrovic A, Tran H, Drew C A and Johnston G A R 1994 Chirality 6 583
Yuan C and Li C 1993 Tetrahedron Lett. 34 5959
Blades K, Lequeux T P and Percy J M 1996 Chem. Commun. 1457
Blades K, Butt A H, Cockerill G S, Easterfield H J, Lequeux T P and Percy J M 1999 J. Chem. Soc. Perkin Trans. 1 3609
Krawczyk H, Wolf W M and Sliwinski M 2002 J. Chem. Soc. Perkin Trans. 1 24 2794
Midura W H and Krysiak J A 2004 Tetrahedron 60 12217
Krawczyk H, Albrecht Ł, Wojciechowski J and Wolf W M 2006 Tetrahedron 62 9135
Kraus G A and Goronga T 2007 Synthesis 1765
Albrecht A, Albrecht L, Rozalski M, Krajewska U, Janecka A, Studzian K and Janecki T 2010 New J. Chem. 34 750
Garzon C, Attolini M and Maffei M 2011 Synthesis 3109
Rai V, Mobin S M and Namboothiri I N N 2007 Tetrahedron: Asymmetry 18 2719
Rai V and Namboothiri I N N 2008 Tetrahedron: Asymmetry 19 767
Hu K, Liu T, Lu A D, Liu Y, Wang Y, Wu G, Zhou Z and Tang C 2011 Eur. J. Org. Chem. 3507
Tripathi C B, Kayal S and Mukherjee S 2012 Org. Lett. 14 3296
Chen Z, Matsunaga S and Shibasaki M 2009 Synlett 1635
Xue Z-Y, Li Q-H, Tao H-Y and Wang C-J 2011 J. Am. Chem. Soc. 133 11757
Acknowledgements
INNN thanks the Department of Science and Technology (DST), India, and Board of Studies in Nuclear Sciences (BRNS), India, for financially supporting research in the area of amino/nitrophosphonates. KB thanks the Council of Scientific and Industrial Research (CSIR) for a Senior Research Fellowship and DN thanks DST for a Postdoctoral Fellowship. The authors thank Prof. Mahesh K Lakshman, The City University of New York, for his encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Abbreviations
Abbreviations
- APCPr:
-
1-Amino-2-phosphonomethylcyclo- propanecarboxylic acid
- N-AcDMPT:
-
N-Acetyldemethyl phosphinothricin
- N-AcDMPTT:
-
N-Acetyldemethyl phosphinothricin tripeptide
- S-BINAM:
-
(S)-(-)-1,1′-Binaphthalene-2,2′-diamine
- CDI:
-
1,1′-Carbonyldiimidazole
- DDQ:
-
2,3-Dichloro-5,6-dicyano-1, 4-benzoquinone
- DIAD:
-
Diisopropyl azodicarboxylate
- DMAP:
-
4-Dimethylaminopyridine
- DMP:
-
Dess–Martin periodinane
- DMPU:
-
N,N′-Dimethyl-N,N′-trimethyl-eneurea
- DPPA:
-
Diphenylphosphoryl azide
- GABA:
-
γ-Aminobutyric acid
- GAPA:
-
γ-Aminophosphonic acid
- HMPA:
-
Hexamethylphosphoramide
- LDA:
-
Lithium di-isopropylamide
- LiHMDS:
-
Lithium hexamethyldisilazide
- MPLC:
-
Medium pressure liquid chromatography
- NMM:
-
N-Methylmorpholine
- NaHMDS:
-
Sodium hexamethyldisilazide
- PCC:
-
Pyridinium chlorochromate
- PTSA:
-
p-Toluene sulphonic acid
- TBAF:
-
Tetra-n-butylammonium fluoride
- TBDMS:
-
Tert-butyldimethylsilyl
- THF:
-
Tetrahydrofuran
- TMEDA:
-
Tetramethylethylenediamine
- TMSBr:
-
Trimethylsilyl bromide
- TMSI:
-
Trimethylsilyl iodide
- TMSN3 :
-
Trimethylsilyl azide
Rights and permissions
About this article
Cite this article
BERA, K., NADKARNI, D. & NAMBOOTHIRI, I.N.N. Asymmetric synthesis of \(\boldsymbol{\gamma}\)-aminophosphonates: The bio-isosteric analogs of \(\boldsymbol{\gamma}\)-aminobutyric acid. J Chem Sci 125, 443–465 (2013). https://doi.org/10.1007/s12039-013-0418-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0418-6