Abstract
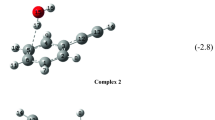
Hydrogen-bonded complexes of phenylacetylene (PhAc) with methanol (MeOH) and diethylether (DEE) were studied using matrix isolation infrared spectroscopy. This study specifically searched for the ≡C-H ⋯O hydrogen bonded complex in these systems, which manifest a n-σ* interaction and which is a local minimum on the PhAc-MeOH potential surface, as in the case of PhAc-H2O heterodimer. This n-σ* local minimum eluded observation in gas phase studies and it was therefore thought interesting to look for this isomer in cryogenic matrices. While MeOH can interact with PhAc as both a proton donor (O-H ⋯π complex) or a proton acceptor (n-σ* complex), DEE can only manifest the n-σ* isomer. A comparison of the spectral shifts observed in the features of PhAc-MeOH and PhAc-DEE would therefore independently confirm the existence or not of n-σ* complex in both these systems. In addition to the n-σ* complex observed in both the above systems, the O-H ⋯π complex was also discerned in the PhAc-MeOH system. These complexes have stabilization energy in the range of 8-25 kJ /mol. The experimental results were corroborated by computations performed at MP2 and M06-2X, levels of theory, using 6-311 ++G(d,p) and aug-cc-pVDZ basis sets. Single point calculations at the CCSD level of theory were also performed. Atoms-in-molecules (AIM), NBO and LMOEDA analysis were also performed to understand the nature of the intermolecular interactions in these complexes.

The ≡C-H..O hydrogen bonded complex in phenylacetylene-methanol system, which is a n-σ* interaction and a local minimum was observed in a cryogenic inert gas solid. This n-σ* local minima had eluded observation in gas phase studies.







Similar content being viewed by others
References
Zhao H, Jiamin Chang J and Du L 2016 Comput. Theor. Chem. 1084 126
Arunan E et al. 2011 Pure Appl. Chem. 83 1619
Grant J H and Legon A C 2015 Phys. Chem. Chem. Phys. 17 858
Arunan E and Mani D 2015 Faraday Discuss 177 51
Nishio M, Hirota M and Umezawa Y 1998 The CH /π interaction, Evidence, Nature and Consequences (New York: Wiley-VCH)
Matsuura H, Yoshida H, Hieda M, Yamanaka S, Harada T, Shin-ya K and Ohno K 2003 J. Am. Chem. Soc. 125 13910
Matsuura H, Yoshida H, Hieda M, Yamanaka S, Harada T, Shin-ya K and Ohno K 2003 J. Am. Chem. Soc. 125 13910
Engdahl A and Nelander B 1985 J. Chem. Phys. 89 2860
Jose K V J, Gadre S R, Sundararajan K and Viswanathan K S 2007 J. Chem. Phys. 127 104501
Viswanathan K S, Sankaran K and Sundararajan K 2000 In Encyclopedia of Analytical Chemistry J B Myers (Ed.) (New York: Wiley)
Whittle E, Dows D A and Pimentel G C 1954 J. Chem. Phys. 22 1943
Karir G and Viswanathan K S 2016 J. Mol. Struct. 1107 145
Singh P C, Bandyopadhyay B and Patwari G N 2008 J. Phys. Chem. A 112 3360
Goswami M and Arunan E 2011 Phys. Chem. Chem. Phys. 13 14153
Singh P C and Patwari G N 2008 J. Phys. Chem. A 112 5121
Venkatesan V, Sundararajan K and Viswanathan K S 2002 J. Phys. Chem. A 106 7707
Kar B P, Ramanathan N, Sundararajan K and Viswanathan K S 2012 J. Mol. Struct. 1024 84
Sundararajan K and Viswanathan K S 2006 J. Mol. Struct. 798 109
Saini J and Viswanathan K S 2016 J. Mol. Struct. 1118 147
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Peterson G A, Montgomery J A, Raghavachari K, Al-Laham M A, Zakrzewski V G, Ortiz J V, Foresman J B, Cioslowski J, Stefanov B B, Nanayakkara A, Challacombe M, Peng C Y, Ayala P Y, Chen W, Wong M W, Andres J L, Replogle E S, Gomperts R, Martin R L, Fox D J, Binkley J S, Defrees D J, Baker J, Stewart J P, Head-Gordon M, Gonzalez C and Pople J A 2010 GAUSSIAN 09 Revision C.01 (Gaussian Inc.: Wallingford CT)
The spectra were simulated using SYNSPEC made available by Irikura K, 1995 National Institute of Standards and Technology, Gaithesburg, MD 20899, USA
Boys S F and Bernardi F 1970 Mol. Phys. 19 553
Blieger-König F, Bayles D and Schönbohn J AIM2000 (Version 1.0): Chemical Adviser: Bader R F W
Glendening E D, Carpenter J E, and Weinhold F, NBO (Version 3.1)
Su P F and Li H 2009 J. Chem. Phys. 131 014102
Schmidt M W, Baldridge K K, Boatz J A, Elbert S T, Gordon M S, Jensen J H, Koseki S, Matsunaga N, Nguyen K A, Su S, Windus T L, Dupuis M and Montgomery J A 1993 J. Comp. Chem. 14 1347
King G W and So S P 1970 J. Mol. Spectrosc. 36 468
Stearns J A and Zwier T S 2003 J. Phys. Chem. A 107 10717
Venkatesan V, Sundararajan K, Sankaran K and Viswanathan K S 2002 Spectrochim. Acta Part A 58 467
Bader R F W 1994 In Atoms in Molecules: A Quantum Theory (Oxford: Clarendon Press)
Koch U and Popelier P L A 1995 J. Phys. Chem. 99 9747
Popelier P L A 2000 In Atoms in Molecules: An introduction (London: Prentice Hall)
Acknowledgements
GK and MF gratefully acknowledge the fellowship from DST and MHRD, respectively. The authors are also grateful to IISER Mohali, India for the facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
The electronic supporting information can be seen at www.ias.ac.in/chemsci.
Special Issue on CHEMICAL BONDING
Celebrating 100 years of Lewis Chemical Bond
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KARIR, G., FATIMA, M. & VISWANATHAN, K.S. The elusive ≡C-H⋯O complex in the hydrogen bonded systems of Phenylacetylene: A Matrix Isolation Infrared and Ab Initio Study. J Chem Sci 128, 1557–1569 (2016). https://doi.org/10.1007/s12039-016-1166-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1166-1