Abstract
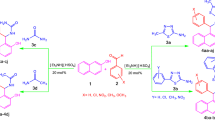
An efficient and inexpensive synthesis of N-substituted amides from the reaction of aliphatic and aromatic nitriles with various benzylic alcohols (secondary and tertiary) and tert-butyl alcohol by refluxing nitromethane via the Ritter reaction catalyzed by aluminum hydrogen sulfate [Al(HSO4)3] is described. The catalyst which is an air-stable, cost-effective solid acid could be readily recycled by filtration and reused four times without any significant loss of its activity.

An efficient and inexpensive synthesis of N-substituted amides by refluxing nitromethane via the Ritter reaction catalyzed by aluminum hydrogen sulfate [Al(HSO4)3] is described. The catalyst which is an air-stable, cost-effective solid acid could be readily recycled by filtration and reused four times without any significant loss of its activity.


Similar content being viewed by others
References
(a) Ritter J J and Minieri P P 1948 J. Am. Chem. Soc. 70 4045; (b) Benson F R and Ritter J J 1949 J. Am. Chem. Soc. 71 4128; (c) Krimen L I and Cota D J 1969 Org. React. 17 213
Brewer A R E 2007 In Name Reactions for Functional Group Transformations Li J J and Corey E J (Ed.) (John Wiley & Sons: Hoboken) p. 471
Olah G A, Yamato T, Iyer P S, Trivedi N J, Singh B P and Surya Prakash G K 1987 Mater. Chem. Phys. 17 21
Yamato T, Hu J Y and Shinoda N 2007 J. Chem. Res. 11 641
Polshettiwar V and Varma R S 2008 Tetrahedron Lett. 49 2661
Gullickson G C and Lewis D E 2003 Synthesis 681
Subba Reddy B V, Sivasankar Reddy N, Madan C. and Yadav J S 2010 Tetrahedron Lett. 51 4827
Sanz R, Martínez A, Guilarte V, Álvarez-Gutiérrez J M and Rodríguez F 2007 Eur. J. Org. Chem. 2007 4642
Ma’mani L, Heydari A and Sheykhan M 2010 Appl. Catal. A. 384 122
Mohammadi Ziarani G., Badiei A R, Dashtianeh Z, Gholamzadeh P and Mohtasham N 2013 Res. Chem. Intermed. 39 3157
Tamaddon F, Khoobi M and Keshavarz E 2007 Tetrahedron Lett. 48 3643
Aoyama T, Kobayashi T, Takido T and Kodomari M 2014 Synlett 25 2365
Rapolu R K, Naba Mukul B, Bommineni S R, Potham R, Mulakayala N and Oruganti S 2013 RSC Adv. 3 5332
Norell J R 1970 J. Org. Chem. 35 1611
Chen H G, Goel O P, Kesten S and Knobelsdorf J 1996 Tetrahedron Lett. 45 8129
Baum J C, Milne J E, Murry J A and Thiel O R 2009 J. Org. Chem. 74 2207
Martinez A G, Alvarez R M, Vilar E T, Fraile A G, Hanack M and Subramanian L R 1989 Tetrahedron Lett. 30 581
Khaksar S and Fattahi E 2011 Tetrahedron Lett. 52 5943
Jiang S, Wang Z, Jiang Z, Li J, Zhou S and Pu L 2012 Lett. Org. Chem. 9 24
Callens E, Burton A J and Barrett A G M 2006 Tetrahedron Lett. 47 8699
Theerthagiri P, Lalitha A and Arunachalam P N 2010 Tetrahedron Lett. 51 2813
Yaragorla S, Singh G, Saini P L and Reddy M K 2014 Tetrahedron Lett. 55 4657
Al-huniti M H and Lepore S D 2013 Adv. Synth. Catal. 355 3071
(a) Top S and Jaouen G 1981 J. Org. Chem. 46 78; (b) Barton D H R, Magnus P D and Young R N 1973 J. Chem. Soc., Chem. Commun. 331; (c) Mukhopadhyay M, Reddy M M, Maikap G G and Iqbal J 1995 J. Org. Chem. 60 2670
Kalkhambkar R G, Waters S N and Laali K K 2011 Tetrahedron Lett. 52 867
Jiang F, Lin Y J, Duan H F, Li Z H, Cao J G and Liang D P 2010 Chem. Res. Chin. Univ. 26 384
Olah G A, Balaram Gupta B G and Narang S C 1979 Synthesis 274
Ibrahim N, Hashmi A S K and Rominger F 2011 Adv. Synth. Catal. 353 461
Shakeri M S, Tajik H and Niknam K. 2012 J. Chem. Sci. 124 1025
Gawande M B, Rathi A K, Nogueira I D, Varma R S and Branco P S 2013 Green Chem. 15 1895
Mokhtary M and Goodarzi G 2012 Chin. Chem. Lett. 23 293
Niknam K, Zolfigol M A and Sadabadi T 2007 J. Iran. Chem. Soc. 4 199
Sadeghi B, Farahzadi E and Hassanabadi A 2012 J. Chem. Res. 36 539
Dokli I and Gredičak M 2015 Eur. J. Org. Chem. 2015 2727
Barbero M, Bazzi S, Cadamuro S and Dughera S 2009 Eur. J. Org. Chem. 2009 430
Katkar K V, Chaudhari P S and Akamanchi K G 2011 Green Chem. 13 835
Jefferies L R and Cook S P 2014 Tetrahedron Lett. 70 4204
Salehi P and Rostamian M A 2000 Synth. Commun. 30 671
Maki T, Ishihara K and Yamamoto H 2006 Org. Lett. 8 1431
Maki T, Ishihara K and Yamamoto H 2007 Tetrahedron 63 8645
Niknam K., Zolfigol M A, Shayeghb M and Zareb R 2007 J. Chin. Chem. Soc. 54 1067
Xufeng L, Wang J, Fangxi X and Yanguang W 2009 J. Chem. Res. 10 638
Bakibaev A A, Tignibidina L G, Filimonov V D, Pustovoitov A V, Gorshkova V K, Saratikov A S and Krasnov V A 1989 Pharm. Chem. J. 23 978
Hazarikaa N, Baishya G and Phukan P 2015 Synthesis 47 2851
Khalafi-Nezhad A, Foroughi H O, Doroodmand M M and Panahi F 2011 J. Mater. Chem. 21 12842
Salehi P, Khodaei M M, Zolfigol M A and Keyvan A 2001 Synth. Commun. 31 1947
Agwada V C 1984 J. Chem. Eng. Data. 29 231
Lasslo A and Jordan W D 1956 J. Org. Chem. 21 805
Weibin S, Lei M and Lihong H 2011 Synth. Commun. 41 3186
Martinelli J R, Clark T P, Watson D A, Munday R H and Buchwald S L 2007 Angew. Chem. Int. Ed. 46 8460
Zhang M, Imm S, Baehn S, Neubert L, Neumann H and Beller M 2012 Angew. Chem. Int. Ed. 51 3905
Debnath P, Baeten M, Lefvre N, Van Daele S and Maes B U W 2015 Adv. Synth. Catal. 357 197
Sanguigni J A and Levine R 1964 J. Med. Chem. 7 573
Neale R S, Marcus N L and Schepers R G 1966 J. Am. Chem. Soc. 88 3051
Khafajeh S, Akhlaghinia B, Rezazadeh S and Eshghi H 2014 J. Chem. Sci. 126 1903
NazariMoghadam B, Akhlaghinia B and Rezazadeh S 2015 Res. Chem. Intermed. DOI: 10.1007/s11164-015-2098-y
(a) Razavi N and Akhlaghinia B 2015 RSC Adv. 5 12372; (b) Razavi N and Akhlaghinia B 2016 New J. Chem. 40 447; (c) Zarghani M and Akhlaghinia B 2015 Appl. Organometal. Chem. 29 683; (d) Zarghani M and Akhlaghinia B 2015 RSC Adv. 5 87769; (e) Ghodsinia E S S and Akhlaghinia B 2015 RSC Adv. 5 49849; (f) Zareie Z and Akhlaghinia B 2015 Chem. Pap. 69 1421; (j) Jahanshahi R and Akhlaghinia B 2015 RSC Adv. 5 104087
Acknowledgements
The authors gratefully acknowledge the partial support of this study by Ferdowsi University of Mashhad Research Council (Grant no. p/ 3/33741).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KARIMIAN, E., AKHLAGHINIA, B. & E GHODSINIA, S.S. An efficient and convenient synthesis of N-substituted amides under heterogeneous condition using Al(HSO4)3 via Ritter reaction. J Chem Sci 128, 429–439 (2016). https://doi.org/10.1007/s12039-016-1036-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1036-x