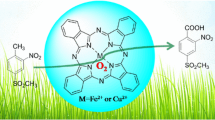
Abstract
Two iron(III) tetraphenyl porphyrin catalytic units are connected by an azo-link to form the dimeric compound A. The compound A was then reacted with Pd 2+ to make a tetrameric iron(III) porphyrin complex B with all four iron(III) catalytic sites open to the substrates and reactants. Both the compounds were characterized spectroscopically and the results of homogeneous oxidation of some alkanes and alkenes with t-BuOOH in presence of catalytic quantities of A and B have indicated remarkable improvement in selectivity and efficiency of A over the monomeric catalyst and B over A.

New tetrameric soluble iron(III) porphyrins and their remarkable catalytic oxidation of several alkenes to alkene oxides by t-BuOOH are explored.



Similar content being viewed by others
References
(a) Groves J T and Han Y-Z 1995 In Cytochrome P 450: Structure, Mechanism and Biochemistry P R Ortiz de Montellano (Ed.) 2nd edn. (New York: Plenum Press) p. 1; (b) Dolphin D and Traylor T G 1997 Acc. Chem. Res. 36 251; (c) Traylor T G, Kim C, Richards J L, Xu F and Perrin C L 1995 J. Am. Chem. Soc. 117 3468
(a) Traylor P S, Dolphin D, and Traylor T G 1984 J. Chem. Soc., Chem. Commun. 279; (b) Traylor T G and Tsuchiya Shinji 1987 Inorg. Chem. 26 1338; (c) Mclain J L, Lee J and Groves J T 2009 In Biomimetic Oxidations by Transition Metal Complexes B Meunier (Ed.) (London: Imperial College Press) p. 91; (d) Que L and Tolman W B 2008 Nature 455 333; (e) Agarwala A and Bandyopadhyay D 2006 Chem. Commun. 4823
(a) Traylor T G, Tsuchiya S, Byun Y S and Kim C 1993 J. Am. Chem. Soc. 115 2775; (b) Traylor T G and Xu F 1990 J. Am. Chem. Soc. 112 178; (c) Bartoli J F, Barch K L, Palacio M, Battioni P and Mansuy D 2001 Chem. Commun. 1718; (d) Suzuki N, Higuchi T and Nagano T 2002 J. Am. Chem. Soc. 124 9622; (e) Traylor T G, Fann W P and Bandyopadhyay D 1989 J. Am. Chem. Soc. 111 8010
(a) Wadhwani P, Mukherjee M and Bandyopadhyay D 2001 J. Am. Chem. Soc. 123 12430; (b) Derat E, Kumar Hirao H and Shaik S 2006 J. Am. Chem. Soc. 128 473; (c) Alkordi M H, Liu Y, Larsen R W, Eubank J F and Eddaoudi M 2008 J. Am. Chem. Soc. 130 12639; (d) Kamaraj K and Bandyopadhyay D 1997 J. Am. Chem. Soc. 119 8099
Che C, Lo V K, Zhou C and Huang J 2011 Chem. Soc. Rev. 40 1950
(a) Merlau ML, Mejia MP, Nguyen S T and Hupp J T 2001 Angew. Chem. Int. Ed. 40 4239; (b) Sheng X, Guo H, Qin Y, Wang X and Wang F 2015 RSC Adv. 5 31664; (c) Lu G, Yang H, Zhu Y, Huggins T, Ren Z J, Liu Z and Zhang W J 2015 Mater. Chem. A 3 4954
(a) Hilal H S, Jondi W, Khalaf S, Keilani A, Suleiman M and Schreine A F 1996 J. Mol. Catal. A: Chem. 113 35; (b) Vinodu M V and Padmanabhan M 2001 Proc. Indian Acad. Sci. (J. Chem. Sci.) 1131
(a) Shultz A M, Farha O K, Hupp J T and Nguyen S T 2011 Chem. Sci. 2 686; (b) Lee K Y, Lee YS, Kim S, Ha H M, Bae S, Huh S, Janga H G and Lee S J 2013 Cryst. Eng. Comm. 15 9360
(a) Sigen A, Zhang Y, Li Z, Xia H, Xue M, Liu X and Mu Y 2014 Chem. Commun. 50 8495; (b) Maeda C, Taniguchi T, Ogawa K and Ema T 2015 Angew. Chem. Int. Ed. 54 134
(a) Singh M K and Bandyopadhyay D 2014 J. Chem. Sci. 126 1707; (b) Liu X, Sigen A, Zhang Y, Luo X, Xia H, Li H and Mu Y 2014 RSC. Adv. 4 6447
Chen X, Huang N, Gao J, Xu H, Xu F and Jiang D 2014 Chem. Commun. 50 6161
Cope A C and Siekman R W 1965 J. Am. Chem. Soc. 87 3272
The monomer (C 44 H 27 N 2 O 2FeCl = 748.45) is supposed to give the parent ion peak at ∼748 but we are getting it at ∼713 which is the parent ion peak – one chloride ion (figure S3). The monomeric porphyrin (figure S4, C 44 H 29 N 5 O 2 [M] ∼659) gives the major peak at [M + H] += 660.2370 as expected
On dissociation of the chloride ions from the dimer A, two + ve charges are generated in the molecule, so expectedly the parent ion peak should come at (M-2Cl)/2 which is clearly visible at 681.1540 (observed), 681.1614 (calculated). We also see the small peak for the dimer at 1361.2811
The tetramer B (Scheme 1) is a gigantic derivative of Cope compound
Mahapatra A K, Bandyopadhyay D, Bandyopadhyay P and Chakravorty A 1986 Inorg. Chem. 25 2214
The crystal structure of well-known Cope compound (I) is given in figure S8. Our present compound B is nothing but a derivative of I. Thus we prepared I and took its ESI mass and have observed the major peak at ∼365 (given in supporting information figure S7). The mass of I (C 24 H 18 N 4Pd 2Cl 2) is 645, but we get the major peak at ∼365 (C 12 H 9 N 2Pd + 2K +). In I the C 12 H 9 N 2Pd fragment has no charge, so it picks up two K + from the K + -formate matrix used during performing the ESI-mass. Thus we conclude that similar thing happens in case of B and because it has already two charges on the iron centres, no additional charge (K +) is required for the major peak (∼733) to appear
Tsuchiya S 1999 J. Am. Chem. Soc. 121 48
(a) Molnar S P and Orchin M J 1969 Organometal. Chem. 16 196; (b) Balch A L and Petridis D 1969 Inorg. Chem. 8 2247
Ahmed A H 2014 Int. J. Chem. Tech. Res. 6 36
Sinha C R, Bandyopadhyay D and Chakravorty A 1988 J. Chem. Soc. Chem. Comm. 468
Acknowledgements
We thank the Department of Science and Technology (DST, India) (Project no. SR/S1/IC-48/2010) for funding and UGC for the fellowship to MKS.
ᅟ
Supplementary Information
Electronic spectra, ESI-Mass, Maldi TOF spectra and stability of the catalysts under oxidizing condition (table S1) are given in supporting information. Supplementary Information is available at www.ias.ac.in/chemsci.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SINGH, M.K., BANDYOPADHYAY, D. A new Organopalladium compound containing four Iron (III) Porphyrins for the selective oxidation of alkanes/alkenes by t-BuOOH. J Chem Sci 128, 383–389 (2016). https://doi.org/10.1007/s12039-016-1032-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1032-1