Abstract
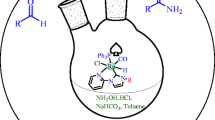
The present contribution describes the synthesis and characterization of a family of robust ruthenium complexes, supported by a tridentate pincer ligand of the type bis-phenolate-N-heterocyclic carbene [ tBu(OCO) 2−] (NHC). Ruthenium(II) complexes (1-3) bearing bis-phenolate-N-heterocyclic carbene ligand were synthesized in good yields by the reaction of imidazolinium proligand (HL) with metal precursors [RuHCl(CO)(EPh3)2(B)] (E = P or As; B = PPh3, AsPh3 or Py) by transmetalation from the corresponding silver carbene complex. All the Ru(II)-NHC complexes have been characterized by elemental analyses, spectroscopic methods as well as ESI mass spectrometry. Based on the spectral results, an octahedral geometry was assigned for all the complexes. The tridentate nature of the tBu(OCO) 2− ligand as well as some level of steric protection provided by the tBu groups may rationalize the excellent stability of the Ru-Ccarbene bond in the present systems. Moreover, for the explorations of catalytic potential of the synthesized compounds, all the three [Ru-NHC] complexes (1-3) were tested as catalysts for amidation of alcohols with amines. Notably, the complex 1 was found to be very efficient and versatile catalyst towards amidation of a wide range of alcohols with amines.

Three new air-stable Ruthenium(II) complexes bearing bis-phenolate-N-heterocyclic carbene ligand were synthesized and characterized by FT-IR, NMR and ESI-Mass. The catalytic study of these complexes towards amidation of alcohol with amines was conducted. This new protocol is effective for many electronically diverse alcohols and amines, providing corresponding amide derivatives in good to excellent yields.



Similar content being viewed by others
References
For reviews, see: (a) Melaimi M, Soleilhavoup M and Bertrand G 2010 Angew. Chem. Int. Ed. 49 8810; (b) Poyatos M, Mata J A and Peris E 2009 Chem. Rev. 109 3677; (c) Hahn F E and Jahnke M C 2008 Angew. Chem. Int. Ed. 47 3122; (c) Herrmann W A 2002 Angew. Chem. Int. Ed. 41 1290; (h) Kumar A and Ghosh P 2012 Eur. J. Inorg. Chem. 3955; (d) Wang F, Liu L J, Wang W, Li S and Shi M 2012 Coord. Chem. Rev. 256 804; (e) Boyarskiy V P, Luzyanin K V and Kukushin V Y 2012 Coord. Chem. Rev. 256 2029
(a) Bantreil X, Schmid T E, Randall R A M, Slawin A M Z and Cazin C S J 2010 Chem. Commun. 46 7115; (b) Scholl M, Ding S, Lee W and Grubbs R H 1999 Org. Lett. 1 953
Samojlowicz C, Bieniek M and Grela K 2009 Chem. Rev. 109 3708
(a) Vicent C, Viciano M, Mas-Marza E, Sanau M and Peris E 2006 Organometallics 25 3713; (b) Poyatos M, Maisse-Francois A, Bellemin-Laponnaz S and Gade L H 2006 Organometallics 25 2634; (c) Lu C, Gu S, Chen W and Qiu H 2010 Dalton Trans. 39 4198; (d) Berding J, Lutz M, Spek A L and Bouwman E 2009 Organometallics 28 1845
Nirmala M, Prakash G, Ramachandran R, Viswanathamurthi P, Malecki J G and Linert W 2015 J. Mol. Catal. A: Chem. 397 56
Nirmala M, Prakash G, Viswanathamurthi P and Malecki J G 2015 J. Mol. Catal. A: Chem. 403 15
(a) Yamaguchi K, Kobayashi H, Wang Y, Oishi T, Ogasawara Y and Mizuno N 2013 Catal. Sci. Technol. 3 318; (b) Chen C and Hyeok Hong S 2011 Org. Biomol. Chem. 9 20; (c) Carey J S, Laffan D, Thomson C and Williams M T 2006 Org. Biomol. Chem. 4 2337
(a) Han S Y and Kim Y A 2004 Tetrahedron 60 2447; (b) Montalbetti C A G N and Falque V 2005 Tetrahedron 61 10827; (c) Valeur E and Bradley M 2009 Chem. Soc. Rev. 38 606; (d) Larock R C 1999 In Comprehensive Organic Transformations (New York: VCH)
(a) Saxon E and Bertozzi C R 2000 Science 287 2007; (b) Damkaci F and DeShong P 2003 J. Am. Chem. Soc. 125 4408; (c) Gololobov Y G and Kasukhin L F 1992 Tetrahedron 48 1353
(a) Ribelin T, Katz C E, English D G, Smith S, Manukyan A K, Day V W, Neuenswander B, Poutsma J L and Aub J 2008 Angew. Chem. Int. Ed. 120 6329; (b) Lang S and Murphy J A 2006 Chem. Soc. Rev. 35 146
(a) Owston N A, Parker A J and Williams J M J 2007 Org. Lett. 9 3599; (b) Hashimoto M, Obora Y, Sakaguchi S and Ishii Y 2008 J. Org. Chem. 73 2894
(a) Martinelli J R, Clark T P, Watson D A, Munday R H and Buchwald S L 2007 Angew. Chem. 119 8612; (b) Nanayakkara P and Alper H 2003 Chem. Commun. 2384
Beller M, Cornils B and Frohning C D 1995 J. Mol. Catal. A: Chem. 104 17
(a) Ali B E and Tijani J 2003 Appl. Organomet. Chem. 17 921; (b) Knapton D J and Meyer T Y 2004 Org. Lett. 6 687; (c) Uenoyama Y, Fukuyama T, Nobuta O, Matsubara H and Ryu I 2005 Angew. Chem. 117 1099; (d) Park J H, Kim S Y, Kim S M and Chung Y K 2007 Org. Lett. 9 2465
(a) Chang J W W and Chan P W H 2008 Angew. Chem. 120 1154; (b) Yoo W J and Li C J 2006 J. Am. Chem. Soc. 128 13064; (c) Cho S, Yoo E, Bae I and Chang S 2005 J. Am. Chem. Soc. 127 16046
(a) Kolakowski R V, Shangguan N, Sauers R R and Williams L J 2006 J. Am. Chem. Soc. 128 5695; (b) Zhang X, Li F, Lu X W and Liu C F 2009 Bio. Conjugate Chem. 20 197
(a) Anastas P and Eghbali N 2010 Chem. Soc. Rev. 39 301; (b) Constable D J C, Dunn P J, Hayler J D, Humphrey J R, Leazer J J L, Linderman R J, Lorenz K, Manley J, Pearlman B A, Wells A, Zaks A and Zhang T Y 2007 Green. Chem. 9 411
(a) Gunanathan C, Ben-David Y and Milstein D 2007 Science 317 790; (b) Naota T and Murahashi S I 1991 Synlett 693; (c) Prechtl M H G, Wobser K, Theyssen N, David Y B, Milstein D and Leitner W 2012 Catal. Sci. Technol. 2 2039; (d) Lanigan R M and Sheppard T D 2013 Eur. J. Org. Chem. 33 7453; (e) Ghosh S C and Hong S H 2010 Eur. J. Org. Chem. 4266; (f) Herrmann W A, Elison M, Fischer J, Kocher C and Artus G R J 1995 Angew. Chem. Int. Ed. Engl. 34 2371; (g) Herrmann W A and Kocher C 1997 Angew. Chem. Int. Ed. 36 2163; (h) Bourissou D, Guerret O, Gabbai F P and Bertrand G 2000 Chem. Rev. 100 39; (i) Hahn F E 2006 Angew. Chem. Int. Ed. 45 1348; (j) Semeril D, Bruneau C and Dixneuf P H 2002 Adv. Synth. Catal. 344 585; (k) Dupont J and Spencer 2004 J. Angew. Chem. Int. Ed. 43 5296; (l) Iglesias M and Albrecht M 2010 Dalton Trans. 39 5213; (m) Araki S, Yokoi K, Sato R, Hirashita T and Setsune J J 2009 Heterocycl. Chem. 46 164; (n) Guisado-Barrios G, Bouffard J, Donnadieu B, Bertrand G 2010 Angew. Chem. Int. Ed. 49 4759; (o) Lalrempuia R, McDaniel N D, Muller-Bunz H, Bernhard S and Albrecht M 2010 Angew. Chem. Int. Ed. 49 9765
(a) Nørdstrom L U, Vogt H and Madsen R 2008 J. Am. Chem. Soc. 130 17672; (b) Watson A J A, Maxwell A C and Williams J M J 2009 Org. Lett. 11 2667; (c) Ghosh S C, Muthaiah S, Zhang Y, Xu X and Hong S H 2009 Adv. Synth. Catal. 351 2643; (d) Zhang Y, Chen C, Ghosh S C, Li Y and Hong S H 2010 Organometallics 29 1374
(a) Fujita K, Takahashi Y, Owaki M, Yamamoto K and Yamaguchi R 2004 Org. Lett. 6 2785; (b) Zweifel T, Naubron J V and Grutzmacher H 2009 Angew. Chem. Int. Ed. 48 559
Shimizu K, Ohshima K and Satsuma A 2009 Chem. Eur. J. 15 9977
(a) Dam J H, Osztrovszky G, Nordstrøm L U and Madsen R 2010 Chem. Eur. J. 16 6820; (b) Makarov I S, Fristrup P and Madsen R 2012 Chem. Eur. J. 18 15683; (c) Maggi A and Madsen R 2012 Organometallics 31 451; (d) Sølvhøj A and Madsen R 2011 Organometallics 30 6044
(a) Watson A J A, Maxwell A C and Williams J M J 2009 Org. Lett. 11 2667; (b) Allen C L and Williams J M J 2011 Chem. Soc. Rev. 40 3405; (c) Allen C L, Chhatwal A R and Williams J M J 2012 Chem. Commun. 48 666; (d) Hamid M H S A, Allen C L, Lamb G W, Maxwell A C, Maytum H C, Watson A J A and Williams J M J 2009 J. Am. Chem. Soc. 131 1766; (e) Watson A J A and Williams J M J 2010 Science 329 635
(a) Ghosh S C, Muthaiah S, Zhang Y, Xu X and Hong S H 2009 Adv. Synth. Catal. 351 2643; (b) Muthaiah S, Ghosh S C, Jee J E, Chen C, Zhang J and Hong S H 2010 J. Org. Chem. 75 3002; (c) Zhang Y, Chen C, Ghosh S C, Li Y and Hong S H 2010 Organometallics 29 1374; (d) Chen C and Hong S H 2011 Org. Biomol. Chem. 9 20; (e) Fu Z, Lee J, Kang B and Hong S H 2012 Org. Lett. 14 6028; (f) Fu Z, Lee J, Kang B and Hong S H 2012 Org. Lett. 146028; (g) Yaşar S, Çekirdek S and Özdemir I 2014 J. Coord. Chem. 67 1236
Ahmed N, Levison J J, Robinson S D and Uttley M F 1974 Inorg. Synth. 15 45
Sanchez-Delgado R A, Lee W Y, Choi S R, Cho Y and Jun M J 1991 Trans. Met. Chem. 16 241
Gopinathan S, Unny I R, Deshpande S S and Gopinathan C 1986 Ind. J. Chem. A. 25 1015
(a) Min K S, Weyhermuller T, Bothe E and Wieghardt K 2004 Inorg. Chem. 43 2922; (b) Bellemin-Laponnaz S, Welter R, Brelot L and Dagorne S J 2009 J. Organomet. Chem. 694 604
(a) Hu X, Castro-Rodriguez I, Olsen K and Meyer K 2004 Organometallics 23 755; (b) Herrmann W A, Schneider S K, Ofele K, Sakamoto M and Herdtweck E 2004 J. Organomet. Chem. 689 2441; (c) Mayr M, Wurst K, Ongania K H and Buchmeiser M R 2004 Chem. Eur. J. 10 1256
Waltman A W and Grubbs R H 2004 Organometallics 23 3105
(a) Csabai P and Joo F 2004 Organometallics 23 5640; (b) Lemke J and Metzler-Nolte N 2008 Eur. J. Inorg. Chem. 21 3359; (c) Maishal T K, Basset J M, Boualleg M, Coperet C, Veyre L and Thieuleux C 2009 Dalton Trans. 35 6956
Pozo C D, Iglesias M and Sanchez F 2011 Organometallics 30 2180
(a) Poyatos M, Mas-Marza E, Sanau M and Peris E 2004 Inorg. Chem. 43 1793; (b) Buchmeiser M R, Wang D, Zhang Y, Naumov S and Wurst K 2007 Eur. J. Inorg. Chem. 3988; (c) Baya M, Eguillor B, Esteruelas M A, Olivan M and Onate E 2007 Organometallics 26 6556; (d) Poyatos M, McNamara W, Incarvito C, Clot E, Peris E and Crabtree R H 2008 Organometallics 27 2128; (e) Ghattas W, Muller-Bunz H and Albrecht M 2010 Organometallics 29 6782
Gnanaprakasam B and Milstein D 2011 J. Am. Chem. Soc. 133 1682
Xie X and Huynh H V 2015 ACS Catal. 5 4143
Acknowledgements
The authors express their sincere thanks to Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, for financial support for this work under the DST FAST TRACK Scheme (No. SR/FT/CS-66/2011). One of the authors (MN) thanks DST-SERB for the award of fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Representative NMR (1H, 13C, 31P) and ESI-MS spectra of ligand and complexes (figures S1-S10), detailed experimental procedure, spectral data and selected 1H and 13C NMR spectra for amide products (figures S11-S18) are given in the supporting information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
NIRMALA, M., VISWANATHAMURTHI, P. Design and synthesis of ruthenium(II) OCO pincer type NHC complexes and their catalytic role towards the synthesis of amides. J Chem Sci 128, 9–21 (2016). https://doi.org/10.1007/s12039-015-0997-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0997-5