Abstract
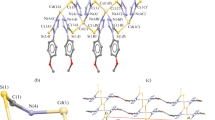
New cadmium complexes of 4-methoxyphenyl dithiophosphinic acids, H3CO-C6 H 4-(R)PS2H were prepared. The five dithiophosphinato ligands (L) involved were of the general structure H3CO-C6 H 4-(R)PS\(_{\mathrm {2}}^{\mathrm {-}}\) with R = 3-methylbutyl, (L1); n-butyl, (L2); 2-methylpropyl, (L3); 1-methylpropyl, (L4) and 2-propyl, (L5). To the best of our knowledge, this is the first report on the preparation and characterization of the n-butyl- derivative. The acid forms of the ligands were obtained by treatment of the Lawesson reagent, (LR) [2,4-bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide] with the corresponding Grignard reagent in dry diethylether. The acids formed were transformed into easily crystallizable ammonium salts (NH4L) for purification. These salts were treated with CdCl2 in ethanol at room temperature to produce the bis-dithiophosphinato cadmium complexes ([Cd(L)2]2) exclusively. The structures of the complexes were elucidated by elemental analysis, MS, FTIR and Raman spectroscopy techniques as well as 1H-, 13C- and 31P- NMR. The crystal structures of [Cd(L1)2]2 and [Cd(L2)2]2 were also studied as examples. X-ray studies confirmed the nonplanar, four-coordination geometry of the complexes and indicate that electron delocalization prevails in the PS\(_{\mathrm {2}}^{\mathrm {-}}\) moiety of the dithiophosphinato groups.

New cadmium 4-methoxyphenyl dithiophosphinato complexes were synthesized and characterized by elemental analysis, mass and FTIR/Raman spectroscopies as well as 1H-, 13C- and 31P- NMR. The crystal structures of [Cd(L1)2]2 and [Cd(L2)2]2 were also elucidated.







Similar content being viewed by others
References
(a) McCleverty J A, Kowalski R S Z, Bailey N A, Mulvaney R and O’Cleirigh D A 1983 J. Chem. Soc. Dalton Trans. 4 627; (b) Satake Y, Kashiwadate K, Iizuka Y, Kouyama T, Katto T and Shiiki Z 1989 US Patent 4826906
(a) Pradip K G and Juthika S 2005 Indian J. Chem. Technol. 12 50; (b) Ziyatdinova G K, Budnikov G K, Samigullin A I, Gabdullina, G T, Sofronov A V, Al’metkina L A, Nizamov I S and Cherkasov R A 2010 J. Anal. Chem. 65 1273; (c) Yagishita K and Konishi S 2013 US Patent 8481467
(a) Artem’ev A V, Malysheva S F, Gusarova N K, Belogorlova N A, Fedorov S V, Timokhin B V, Smirnov V I and Trofimov B A 2012 Chem. Heterocycl. Comp. 483 448; (b) Michalski Jan, Potrzebowski M and Lopusinski A 1984 US Patent 4470933; (c) Kabra V, Mitharwal S and Singh S 2009 Phosphorus, Sulfur Silicon Relat. Elem. 184 2431
Bara A C Silvestru C and Haiduc 1991 Anticancer Res. 11 1651
Bellande E, Comazzi V, Laine J, Lecayon M, Pasqualini R, Duatti A and Hoffschir D 1995 Nucl. Med. and Biol. 22 3 315
Durckheimer W, Bormann D, Ehlers E, Schrinner E and Heymes R 1988 U.S. Patent 4758556
(a) Grigorieva N A, Pashkov G L, Fleitlikh I Y, Nikiforova L K and Pleshkov M A 2010 Hydrometallurgy 105 82; (b) Xihong H, Guoxin T, Jing C and Linfeng R, 2014 Dalton Trans. 43 17352; (c) Wieszczycka K and Zembrzuska J 2014 J. Radioanal. Nucl. Chem. 299 709
(a) Jing C, Meng W, Xuegang L and Jianchen W 2012 Prog. Nucl. Energ. 54 46; (b) Ghoreishi S M, Ansari K and Ghaziaskar H S 2012 J. Supercrit. Fluids 72 288
(a) Karakus M and Yilmaz H 2006 Russ. J. Coord. Chem. 32 6437; (b) Ilyas S N, Gulnara T G, Dmitriy A T, Almaz R N, Ilnar D N and Rafael A C 2014 Phosphorus, Sulfur Silicon Relat. Elem. 189 1354
Wagner J, Ciesielski M, Fleckenstein C A, Denecke H, Garlichs F, Ball A and Doering M 2013 Org. Process Res. Dev. 17 47
(a) Diemert K and Kuchen W 1977 Phosphorus, Sulfur Silicon Relat. Elem. 3 131; (b) Rauhut M M, Currier H A and Wystrach V P 1961 J. Org. Chem. 26 5133
(a) Sağlam E G, Çelik Ö, Yılmaz H and İde S 2010 Transition Met. Chem. 35 399; (b) Yordanov N D, Gochev G, Angelova O and Macicek J 1990 Polyhedron 9 2597; (c) Kuchen W, Metten J and Judat A 1964 Chem. Ber. 97 2306; (d) Cavell R G, Byers W, Day E D and Watkins P M 1972 Inorg. Chem. 11 1598; (e) Daly S R, Keith J M, Batista E R, Boland K S, Kozimor S A, Martin R L and Scott B L 2012 Inorg. Chem. 51 7551
(a) Porta P, Sgamellotti A and Vinciguerra N 1971 Inorg. Chem. 10 541; (b) Pinkerton A A, Ahlers F P, Greiwing H F and Krebs B 1997 lnorg. Chim. Acta 257 77
(a) Calligaris M, Nardin G and Ripamonti A 1970 J. Chem. Soc. A 714; (b) Cavell R G, Day E D, Byers W and Watkins P M 1972 Inorg. Chem. 11 1759; (c) Casas J S, García-Tasende M S, Sánchez A, Sordo J, Castellano E E and Zukerman-Schpector J 1994 Inorg. Chim. Acta 219 115
Byrom C, Malik M A, O’Brien P, White A J P and Williams D J 2000 Polyhedron 19 211
Sağlam E G, Yılmaz H, Dal H and Hökelek T 2012 Phosphorus, Sulfur Silicon Relat. Elem. 187 213
Hoffmann H and Schumacher G 1967 Tetrahedron Lett. 31 2963
Sheldrick G M 2008 Acta Cryst. A64, 112
Farrugia L J 2012 J. Appl. Cryst. 45, 849
International fig for X-Ray Crystallography vol. 4 1974 (Kynoch Press: Birmingham)
(a) Sundee S, Hanlan L and Bernstein J 1975 Inorg. Chem. 14 2012; (b) Czernuszewicz R, Maslowsky E Jr and Nakamoto K 1980 Inorg. Chim. Acta 40 199
Chakravarty M, Pailloux S, Ouizem S, Smith K A, Duesler E N, Paine R T, Williams N J and Hancock R D 2012 Polyhedron 33 327
(a) Keck H and Kuchen W 1983 Phosphorus, Sulfur Silicon Relat. Elem. 14 225; (b) Heinz S, Keck H and Kuchen W 1984 Org. Mass Spoctrom. 19 82; (c) Christoph D, Keck H, Kuchen W, Mathow J and Wunderlich H 1987 Inorg. Chim. Acta 132 213; (d) Mohan P N, Kuchen W, Keck H and Haegele G J 1977 Inorg. Nuclear Chem. 39 833
(a) Karakuş M, Yılmaz H, Bulak E and Lonnecke P 2005 Appl. Organometal. Chem. 19 396; (b) M, Yılmaz H, Özcan Y and İde S 2004 Appl. Organometal. Chem. 18 141
(a) Ramirez R G, Toscano R A, Silverstu C and Haiduc I 1996 Polyhedron 21 3857; (b) Przychodzen W 2004 Phosphorus, Sulfur Silicon Relat. Elem. 179 1621
Bernstein J, Davis R E, Shimoni L and Chang N L 1995 Angew. Chem. Int. Ed. Engl. 34 1555
Acknowledgements
We gratefully acknowledge the financial assistance of Technical Research Council of Turkey (grant nos. TBAG 114Z091, TUBITAK) and Research and Application Centers of Bozok University (BAP2015 FEF/A153). Our thanks also go to the tutors of the Zurich School of Crystallography, for assistance and guidance with the data collection and structure determination of compound [Cd(L1)2]2 and Prof. Davit Zargarian, Chemistry Department of the Université de Montréal for their support with the X-ray facility for [Cd(L2)2]2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
All additional information relating to the characterization of the complexes, namely, ESI-MS (figure 1), IR spectra (figure S2), Raman spectra (figure S3), NMR (1H, 13C and 31P) spectra (figures S4, S5 and S6) are available at www.ias.ac.in/chemsci. Crystallographic data (excluding structure factors) have been deposited with the Cambridge Crystallographic Data Centre as the supplementary publication no. 1060403 (for [Cd(L1)2]2, and CCDC 1060404 (for [Cd(L2)2]2). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: + 44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SAĞLAM, E.G., ACAR, N., SÜZEN, Y. et al. Syntheses and structural characterization of new dithiophosphinato cadmium complexes. J Chem Sci 127, 1653–1663 (2015). https://doi.org/10.1007/s12039-015-0930-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0930-y