Abstract
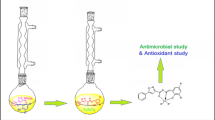
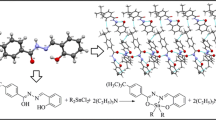
The organotin(IV) complexes [MeSnCl(L)] (2), [BuSnCl(L)] (3), [PhSnCl(L)] (4) and [Me2Sn(L)] (5) were synthesized by reacting organotin(IV) chloride(s) with 5-allyl-2-hydroxy-3-methoxybenzaldehyde-4-thiosemicarbazone [H2L], (1)] in presence of KOH in 1:2:1 molar ratio (metal salt: base:ligand). All the complexes have been characterized by elemental analyses, UV-Vis, FT-IR, 1H, 13C and 119Sn NMR spectral studies. The molecular structure of complex 5 has been confirmed by single crystal X-ray diffraction analysis. The ligand, H2L coordinates to Sn(IV) in thiolate form through phenoxide-O, azomethine-N and thiolate-S atoms. The C-Sn-C angle measured from coupling constant 1 J(119Sn, 13C) for dimethyltin(IV) complex 5 is 123.4 ∘. The 2 J(119Sn, 1H) coupling constant values for complex 2 and 5 are 72.4 and 76.3 Hz, respectively. Proposed geometry for five coordinated Sn(IV) atom is a strongly distorted trigonal bipyramid. Biological studies were preformed in vitro against four bacterial strains which have shown better activities and potential as antibacterial agents.

Organotin(IV) complexes of 5-allyl-2-hydroxy-3-methoxybenzaldehyde-4-thiosemicarbazone were synthesized and characterized by various physico-chemical methods. Crystal structure of dimethyltin(IV) complex was obtained. Ligand (H2L) acts as dinegative ONS tridentate chelating agent, coordinated to tin(IV) atom via phenolic oxygen, azomethine nitrogen and thiolate sulphur atoms. In vitro antibacterial studies revealed that the complexes possess significant activity.





Similar content being viewed by others
References
Campbell M J M 1975 Coord. Chem. Rev. 15 279
Tojal J G, Orad A, Diaz A A, Serra J L, Urtiaga M K, Arriortua M I and Rojo T 2001 J. Inorg. Biochem. 84 271
El-Ayaan U, Youssef M M S and Al-Shihry 2009 J. Mol. Struct. 936 213
Lukmantara A Y, Kalinowski D S, Kumar N and Richardson D R 2013 Bioorg. Med. Chem. Lett. 23 967
Walcourt A, Loyevsky M, Lovejoy D B, Gordeuk V R, Richardson and Des R 2004 Int. J. Biochem. Cell Biol. 36 401
Serda M D, Kalinowski S, Wilczkiewicz A M, Musiol R, Szurko A, Ratuszna A, Pantarat N, Kovacevic Z, Merlot A M, Richardson D R and Polanski R 2012 J. Bioorg. Med. Chem. Lett. 22 5527
Mendes I C, Moreira J P, Speziali N L, Mangrich A S, Takahashi J A and Beraldo H 2006 J. Braz. Chem. Soc. 17 1571
Silva J G, Azzolini L S, Wardell S M S V, Wardell J L and Beraldo H 2009 Polyhedron 28 2301
Khoo L E, Xu Y, Goh N K, Chia L S and Koh L L 1997 Polyhedron 16 573
Dakternieks D, Basu-Baul T S, Dutta S and Tiekink E R T 1998 Organometallics 17 3058
Teoh S G, Ang S H, Fun H K and Ong C W 1999 J. Organomet. Chem. 580 17
Gielen M, Biesemans M and Willen R 2005 Appl. Organomet. Chem. 19 440
Chaudhary P, Swami M, Sharma D K and Singh R V 2009 Appl. Organomet. Chem. 23 140
Zubita J A and Zukerman J J 1987 Inorg. Chem. 24 251
Rebolledo A P, Vieites M, Gambino D, Piro O E, Castellano E E, Zani C L, Souza-Fagundes E M, Teixeira L R, Batista A A and Beraldo H 2005 J. Inorg. Biochem. 99 698
West D X, Bain G A, Butcher R J, Jasinski J P, Li Y, Pozdniakiv R Y, Valdes- Martinez J, Toscano R A and Hernandes-Ortega S 1996 Polyhedron 15 665
Maurya M R, Kumar A, Abid M and Azam A 2006 Inorg. Chim. Acta 359 2439
Alomar K, Khan M A, Allain M and Bouet G 2009 Polyhedron 28 1273
Vieites M, Otero L and Santos D 2009 J. Inorg. Biochem. 103 411
de Sousa G F, Francisco R H P, Gambardella M T d P, Santos R H d A and Abras A 2001 J. Braz. Chem. Soc. 12 722
Hussein M A, Guan T S, Haque R A, Ahamed M B K and Majid A M S A 2015 Polyhedron 85 93
Hussein M A, Guan T S, Haque R A, Ahamed M B K and Abdul Majid A M S 2014 J. Coord. Chem. 67 714
Gómez-Saiz P, García-Tojal J, Maestro M, Mahía J, Lezama L and Rojo T 2003 Eur. J. Inorg. Chem. 2003 2123
López-Torres E, Mendiola M A, Pastor C J and Procopio J R 2003 Eur. J. Inorg. Chem. 2003 2711
Casas J S, García-Tasende M S and Sordo J 2000 Coordination Chemistry Reviews 209 197
Yang Z Y, Yang R D and Yu K B 1996 Polyhedron 15 3771
Garcia-Zarracino R, Ramos-Quinones J and Höpfl H 2003 Inorg. Chem. 42 3835
Tsangaris J M and Williams D R 1992 Appl. Organomet. Chem. 6 3
Sheldrick G M, SHELXTL Version 5.1 Software Reference Manual 1997 Bruker AXS Inc, Madison, Wisconsin, USA
Rahman A, Choudry M A and Thomsen M I W J 2001 In Bioassay Techniques for Drug Development (Harwood Academic Publishers: The Netherlands)
Maurya R M, Jayaswal M N, Puranik V G, Chakrabarti P, Gopinathan S and Gopinathan C 1997 Polyhedron 16 3977
Silverstein R M, Bassler G C and Morrill T C 1981 In Spectrometric Identification of Organic Compounds 4th ed. (Wiley: New York)
Singh S, Bharti N, Naqvi F and Azam A 2004 Eur. J. Med. Chem. 39 459
Costa F F, Rebolledo A P, Matencio T, Calado H D R, Ardisson J D, Cortes M E, Rodrigues B L and Beraldo H 2005 J. Coord. Chem. 58 1307
Mendes I C, Moreira J P, Mangrich A S, Balena S P, Rodrigues B L and Beraldo H 2007 Polyhedron 26 3263
Rajan O A and Chakravarthy A 1981 Inorg. Chem. 20 660
Sedaghat T and Shokohi-Pour Z 2009 J. Coord. Chem. 62 3837
Saraswat B S, Srivastava G and Mehrotra R C 1979 J. Organomet. Chem. 164 153
Teoh S G, Teo S B, Yeap G Y and Declercq J P 1991 Polyhedron 10 2683
Mesubi M A and Enemo R E 1982 Spectrochim. Acta A 38 599
Lockhart T P and Manders W F 1986 Inorg. Chem. 25 892
Nath M, Saini P K and Kumar A 2010 J. Organomet. Chem. 695 1353
Yin H D and Chen S W 2006 Inorg. Chim. Acta 359 3330
Wrackmeyer B 1985 Annual Reports on NMR Spectroscopy 16 73
Chee D N A, Affan M A, Ahmad F B, Asaruddin M R, Sam N, Salam M A, Ismail A and Tan S H 2011 J. Coord. Chemi. 64 4191
de Sousaa G F, Franciscob R H P, Gambardellab M T P, Santosb R H d. and Abras A 2001 Braz. Chem. Soc. 12 722
Labisbal E, Rodriguez L, Sousa-Pedrares A, Alonso M, Vizoso A, Romero J, Garcia-Vazquez A and Sousa A 2006 J. Organomet. Chem. 691 1321
Purcell K F and Kotz J C 1980 In An Introduction to Inorganic Chemistry (Saunders College Publishing: Orlando, USA)
Pettinari C, Marchetti F, Pettinari R, Martini D, Drozdov A and Troyanov S 2001 Inorg. Chim. Acta 325 103
Lopez-Torres E, Mendiola M A, Pastor C J and Procopio J R 2003 Eur. J. Inorg. Chem. 2003 2711
Casas J S, Castineiras A, Rodríguez-Arguelles M C, Sanchez A, Sordo J, Vazquez- Lopez A and Vazquez-Lopez E M 2000 J. Chem. Soc., Dalton Trans. 2000 2267
Rehman W, Baloch M K and Badshah A 2008 Eur. J. Med. Chem. 43 2380
Chohan Z H, Hassan M U I, Khan K M and Supuran C T 2005 J. Enzyme Inhib. Med. Chem. 20 183
Dharmaraj N, Vishwanathamurthi V and Natarajan K 2001 Transition Met. Chem. 26 105
Ming-Xue L, Dong Z, Li-Zhi Z, Jing-Yang N and Bian-Sheng J 2011 J. Organomet. Chem. 696 852
Mendes I C, Moreira J P, Ardisson J D, dos Santos R G, da Silva P R O, Garcia I, Castiñeiras A and Beraldo H 2008 Eur. J. Med. Chem. 43 1454
Shujah S, Rehman Z, Muhammada N, Ali S, Khalid N and Tahir M N 2011 J. Organomet. Chem. 696 2772
Acknowledgment
R.A.H. thanks Universiti Sains Malaysia (USM) for the Research University (RU) Grant 1001/PKIMIA/ 811217. M.A.S. thanks USM and Third World Academy of Sciences (TWAS) for a post-doctoral research fellowship. We also thank the Bangladesh Petroleum Exploration and Production Co. Ltd. (BAPEX), Bangladesh, for the study leave to M. A. Salam.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
UV-Visible, FT-IR and multinuclear NMR (1H, 13C, and 119Sn) spectra of the ligand and its complexes (figures S1–S9) are available at www.ias.ac.in/chemsci. CCDC reference number 1054928 contains the supplementary crystallographic data for [Me2Sn(L)] (5). This data can be obtained free of charge from the Cambridge Crystallographic data center via www.ccdc.ac.uk/data_request/cif or from the Cambridge Crystallographic data center, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
HAQUE, R.A., SALAM, M.A. Synthesis, structural characterization and biological activities of organotin(IV) complexes with 5-allyl-2-hydroxy-3- methoxybenzaldehyde-4-thiosemicarbazone. J Chem Sci 127, 1589–1597 (2015). https://doi.org/10.1007/s12039-015-0924-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0924-9