Abstract
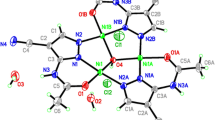
A linear trinuclear cluster of the type [{Zn3(HTrz)6(H2O)6}6+(NO\(_{\mathrm {3}}^{\mathrm {-}}\))6(H2O)] (ZnT) has been synthesized by one-pot reaction between 1,2,4-triazole and Zn(NO3).6H2O. Molecule consists of three Zn2++ ions linearly connected by 1,2,4-triazole with tri-fold symmetry. The coordination geometry around the zinc centre is octahedral with zinc-zinc separation of 3.810 Å. The coordination environment of central Zn2++ ion is satisfied by nitrogen atoms of six 1,2,4-triazoles, while the geometry of terminal Zn2++ ions is fulfilled by nitrogen atoms of three 1,2,4-triazoles and three water molecules. The thermal and absorption properties of ZnThave been reported for the first time.

A rare linear, discrete, hexa-cationic trinuclear zinc triazole cluster has been synthesized and structurally characterized.




Similar content being viewed by others
References
(a) Steed J W and Atwood J L 2013 In Supramolecular Chemistry (New York: John Wiley); (b) Lehn J-M 1995 In Supramolecular Chemistry: Concepts and Perspectives (Weinheim: VCH)
Caulder D L and Raymond K N 1999 Acc. Chem. Res. 32 975
Saalfrank R W and Demleitner B In Perspectives in Supramolecular Chemistry 1999 J P Sauvage (ed.) (Weinheim: Wiley-VCH) Vol. 5 pp. 1–51
Uller E, Demleitner B, Bernt I and Saalfrank R W 2000 In Structure and Bonding M Fujita (ed.) (Berlin: Springer) Vol. 96 p. 149
Moore D S and Robinson S D 1988 Adv. Inorg. Chem. 32 171
Parkin G 2004 Chem. Rev. 104 699
Liu K, Shi W and Cheng P 2011 Dalton Trans. 40 8475
Haasnoot J G 2000 Coord. Chem. Rev. 131 200–202
Reimann C W and Zocchi M 1971 Acta Crystallogr. Sect. B 27 682
Spek A L and Vos G 1983 Acta Cryst. C39 990
Xu W, Jiang F, Zhou Y, Xiong K, Chen L, Yang M, Feng R and Hong M 2012 Dalton Trans. 41 7737
Sheldrick G M 1990 Acta Crystallogr. Sect A 46 467
Sheldrick G M SHELXL-97, Program for Crystal Structure Refinement, 1997 (Göttingen: Universität Göttingen)
Grirrane A, Resa I, Rodríguez A and Carmona E 2008 Coord. Chem. Rev. 252 1532
Acknowledgements
We thank the DST, New Delhi (SR/FT/CS94/2010) and IIT Hyderabad for the financial support. NB thanks UGC for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
FT-IR spectrum and CheckCIF report are available free of charge via the Internet at www.ias.ac.in/chemsci. CCDC 944805 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BABU, C.N., SURESH, P., SATHYANARAYANA, A. et al. Crystal structure and solid-state properties of discrete hexa cationic trinuclear zinc triazole cluster. J Chem Sci 127, 1369–1373 (2015). https://doi.org/10.1007/s12039-015-0901-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0901-3