Abstract
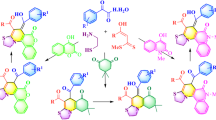
Various N-aryl-2-aminobenzoxazoles and N-aryl-2-aminobenzothiazoles were synthesized from o-aminophenol and o-aminothiophenol, respectively, mediated by dithiocarbamate in one step. The salient features of this method include mild reaction condition, high yield and large scale synthesis. Application of this methodology has been demonstrated by synthesizing potent Aurora kinase-A inhibitors.

Various N-aryl-2-aminobenzoxazoles and N-aryl-2-aminobenzothiazoles were synthesized from o-aminophenol and o-aminothiophenol, respectively, mediated by dithiocarbamate in one step. Application of this methodology has been demonstrated by synthesizing potent Aurora kinase-A inhibitors.


Similar content being viewed by others
References
(a) Harris N V and Fenton G 2002 World Pat. 2002098426; (b) Darque A, Dumetre A, Hutter S, Casano G, Robin M, Pannecouque C and Azas N 2009 Bioorg. Med. Chem. Lett. 19 5962
Martin R E, Mohr P, Maerki H P, Guba W, Kuratli C, Gavelle O, Binggeli A, Bendels S, Alvarez-Sanchez R, Alker A, Polonchuk L and Christ A D 2009 Bioorg. Med. Chem. Lett. 19 6106
(a) Muro F, Limura S, Yoneda Y, Chiba J, Watanabe T, Masaki S, Takayama G, Yokoyama M, Takashi T, Nakayama A and Machinaga N 2009 Bioorg. Med. Chem. 17 1232; (b) Song H, Oh S R, Lee H K, Han G, Kim J H, Chang H W, Doh K E, Rhee H K and Choo Y P 2010 Bioorg. Med. Chem. 18 7580
Potashman M H, Bready J, Coxon A, DeMelfi Jr. T M, DiPietro L, Doerr N, Elbaum D, Estrada J, Gallant P, Germain J, Gu Y, Harmange J C, Kaufman S A, Kendell R, Kim J L, Kumar G N, Long A M, Neervannan S, Patel V F, Polverino A, Rose P, Plas S ven der, Douglas W, Zanon R and Zhao H 2007 J. Med. Chem. 50 4351
Das J, Moquin R V, Lin J, Doweyko H M, Defex H F, Fung Q, Pang S, Pitt S, Shen D R, Schieven G L, Barrish J C and Wityak J 2003 Bioorg. Med. Chem. Lett. 13 2587
Liu C, Lin J, Pitt S, Zhang R F, Sack J S, Kieter S E, Kish K, Doweyko A M, Zhang H, Marathe P H, Trzaskos J, Mckinnon M, Dodd J H, Barrish J C, Schieven G L and Lefthenis K 2008 Bioorg. Med. Chem. Lett. 18 1874
Carpenter R D, Andrei M, Aina O H, Lau H Y, Lightstone F C, Liu R, Lam K S and Kurth M J 2009 J. Med. Chem. 52 14
(a) Ozaki S 1972 Chem Rev. 72 457; (b) Ogura H, Mineo S and Nakagawa K 1981 Chem. Pharm. Bull. 29 1518; (c) Tian Z, Plata D J, Wittenberger S J and Bhatia A V 2005 Tetrahedron Lett. 46 8341
Qian X H, Li Z B, Song G H and Li Z 2001 J. Chem. Res. Synop. 4 138
Ogura H, Mineo S and Nakagawa K 1981 Chem. Pharm. Bull. 29 1518
Cee V J and Downing N S 2006 Tetrahedron Lett. 47 3747
Yella R and Patel B K 2010 J. Comb. Chem. 12 754
(a) Qui J W, Zhang X G, Tang R Y, Zhong P and Li J H 2009 Ad. Synth. Catal. 351 2319; (b) Wang J, Peng F, Jiang J L, Lu Z J, Wang L Y, Bai J and Pan Y 2008 Tetrahedron Lett. 49 467; (c) Joyce L L, Evindar G and Batey R A 2004 Chem. Comm. 446; (d) Benedı C, Bravo F, Uriz P, Fernández E, Claver C and Castillón S 2003 Tetrahedron Lett. 44 6073
Joyce L L and Batey R A 2009 Org. Lett. 11 2792
(a) Das P, Kirankumar C, Nareshkumar K, Innus Md, Iqbal J and Srinivas N 2008 Tetrehedron Lett. 49 922; (b) Nareshkumar K, Sreeramamurthy K, Sandananda P, Mukkanti K and Das P 2010 Tetrahedron Lett. 51 899
(a) Azizi N, Ebrahimi F, Aakbari E, Aryansab F and Saidi M R 2007 Synthesis 2797; (b) Azizi N, Aryanasab F, Torkiyan L, Ziyaei A and Saidi M R 2006 J. Org. Chem. 71 3634; (c) Azizi N, Aryanasab F and Saidi M R 2006 Org. Lett. 8 5275; (d) Wang Y Q, Ge Z M,Hou X L, Cheng T M and Li R T 2004 Synthesis 675; (e) Salvatore R N, Sahab S and Jung K 2001 Tetrahedron Lett. 42 2055
(a) Ple P A, Green T P, Hennequin L F, Curwen J, Fennel M, Allen J, van der Brempt C L and Costello G 2004 J. Med. Chem. 47 871; (b) Sreeramamurthy K, Ashok E, Mahendar V, Santoshkumar G and Das P 2010 Synlett. 721
Pollard J R and Mortimore M 2009 J. Med. Chem. 52 2629
Acknowledgments
The authors are very thankful to analytical department of Dr. Reddy’s Laboratories Ltd. for the spectral and analytical data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
NMR, IR and mass spectra of the synthesized compounds are reported in Supplementary Information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KATARI, N.K., VENKATANARAYANA, M. & SRINIVAS, K. Dithiocarbamate promoted practical synthesis of N-Aryl-2-aminobenzazoles: Synthesis of novel Aurora-A kinase inhibitor. J Chem Sci 127, 447–453 (2015). https://doi.org/10.1007/s12039-015-0803-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0803-4