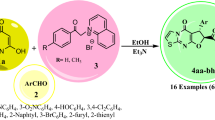
Abstract
A novel class of medicinally important 4-aryl-substituted dihydropyrimidine derivatives fused to pyrazole and triazole scaffolds has been found which is obtained via a newly designed regioselective domino reaction using tungstate sulfuric acid (TSA) as a Brønsted acid recyclable catalyst. Throughout this tri-component tandem reaction, 4-aryl-substituted dihydropyrimidine derivatives fused to pyrazole and triazole moieties were regioselectively formed under environmentally benign solvent-free conditions with high yields. By this achievement, some novel potentially interesting biologically active products were synthesized via a green and eco-friendly procedure.
Graphical abstract




Similar content being viewed by others
References
Dömling A, Ugi IA (2000) Angew Chem Int Ed 39:3168
Dömling A, Wang W, Wang K (2012) Chem Rev 112:3083
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Chem Rev 114:8323
Tamaddon F, Farahi M, Karami B (2011) J Mol Catal A Chem 337:52
Eskandari K, Karami B, Khodabakhshi S (2014) J Chem Res 38:600
Eskandari K, Karami B, Khodabakhshi S, Farahi M (2015) Lett Org Chem 12:38
Liao WL, Li SQ, Wang J, Zhang ZY, Yang ZW, Xu D, Xu C, Lan HT, Chen ZZ, Xu ZG (2016) ACS Comb Sci 18:65
Eskandari K, Karami B (2016) Monatsh Chem 147:2119
De Coen LM, Heugebaert TSA, Garcia D, Stevens CV (2016) Chem Rev 116:80
Kralisch D, Ott D, Gericke D (2015) Green Chem 17:123
Wenda S, Illner S, Mell A, Kragl U (2011) Green Chem 13:3007
Singh MS, Chowdhury S (2012) RSC Adv 2:4547
Karami B, Farahi M, Pam F (2014) Tetrahedron Lett 55:6292
Ralphs K, Hardacre C, James SL (2013) Chem Soc Rev 42:7701
Feng J, He Y, Liu Y, Dua Y, Li D (2015) Chem Soc Rev 44:5291
Karami B, Eskandari K, Gholipour S, Jamshidi M (2013) Org Prep Proced Int 45:220
Karami B, Farahi M, Banaki Z (2015) Synlett 26:1804
Khodabakhshi S, Karami B, Eskandari K, Farahi M (2014) Tetrahedron Lett 55:3753
Poorali L, Karami B, Eskandari K, Azizi M (2013) J Chem Sci 125:591
Kantam ML, Roy M, Roy S, Sreedhar B, Lal-De R (2008) Catal Commun 9:2226
Murkute AD, Jackson JE, Miller DJ (2011) J Catal 278:189
Karami B, Eskandari K, Azizi M (2013) Lett Org Chem 10:722
Farahi M, Karami B, Sedighimehr I, Mohamadi-Tanuraghaj H (2014) Chin Chem Lett 25:1580
Farahi M, Karami B, Azari M (2013) C R Chim 16:1029
Goel N, Drabu S, Afzal O, Bawa S (2014) J Pharm BioAllied Sci 6:253
Bender CM, Merriman JD, Gentry AL, Ahrendt GM, Berga SL, Brufsky AM, Casillo FE, Dailey MM, Erickson KI, Kratofil FM, McAuliffe PF, Rosenzweig MQ, Ryan CM, Sereika SM (2015) Cancer 121:2627
Hertz DL, Barlow WE, Kidwell KM, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, Livingston RB, Gralow J, Hayes DF, Hortobagyi GN, Mehta RS, Rae JM (2016) Br J Clin Pharmacol 81:1134
Da Silva AR, De Andrade-Neto JB, Da Silva CR, Campos-Rde S, Costa-Silva RA, Freitas DD, Nascimento DFB, De Andrade LN, Sampaio LS, Grangeiro TB, Magalhaes HI, Cavalcanti BC, De Moraes MO, Nobre-Junior HV (2016) Antimicrob Agents Chemother 60:3551
Cikla-Süzgün P, Kaushik-Basu N, Basu A, Arora P, Talele TT, Durmaz I, Cetin-Atalay R, Kücükgüzel SG (2015) J Enzyme Inhib Med Chem 30:778
Purohit MN, Panjamurthy K, Elango S, Hebbar K, Mayur YC, Raghavan SC (2011) Nucleosides. Nucleotides Nucleic Acids 30:873
Patel NB, Khan IH (2011) J Enzyme Inhib Med Chem 26:527
Plech T, Wujec M, Kosikowska U, Malm A, Rajtar B, Polz-Dacewicz M (2013) Eur J Med Chem 60:128
Somorai T, Szilagyi G, Reiter J, Pongo L, Lang T, Toldy L, Horvath S (1986) Arch Pharm 319:238
Kaplancikli ZA, Turan-Zitouni G, Ozdemir A, Revail G (2008) Eur J Med Chem 43:155
El-Shehry MF, Abu-Hashem AA, El-Telbani EM (2010) Eur J Med Chem 45:1906
Chen J, Sun XY, Chai KY, Lee JS, Song MS, Quan ZS (2007) Bioorg Med Chem 15:6775
Almajan GL, Barbucenau SF, Almajan ER, Draghici C, Saramet G (2009) Eur J Med Chem 44:3083
Kolos NN, Kovalenko LU, Borovskoy VA (2011) Chem Heterocycl Compd 47:983
Ruisi G, Canfora L, Bruno G, Rotondo A, Mastropietro TF, Debbia EA, Girasolo MA, Megna B (2010) J Organomet Chem 695:546
El-Gendy MMA, Shaaban M, Shaaban KA, El-Bondkly AM, Laatsch H (2008) J Antibiot 61:149
Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2010) Nat Prod Rep 27:165
Rudenko RV, Komykhov SA, Musatov VI, Desenko SM (2009) J Heterocycl Chem 46:285
Magan R, Marin C, Rosales MJ, Salas JM (2005) Pharmacology 73:41
Dandia A, Sarawgi P, Arya K, Khanturia S (2006) Arkivoc XVI:83
Farahi M, Davoodi M, Tahmasebi M (2016) Tetrahedron Lett 57:1582
Eskandari K, Karami B, Farahi M, Mouzari V (2016) Tetrahedron Lett 57:487
Karami B, Eskandari K, Farahi M, Barmas A (2012) J Chin Chem Soc 59:473
Tamaddon F, Farahi M (2012) Synlett 23:1379
Ma N, Jiang B, Zhang GE, Tu SJ, Wever W, Li G (2010) Green Chem 12:1357
Ramalingan C, Park SJ, Lee IS, Kwak YW (2010) Tetrahedron 66:2987
Acknowledgements
The authors would like to gratefully thank Yasouj University Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farahi, M., Karami, B., Banaki, Z. et al. TSA-catalyzed regioselective synthesis of medicinally important 4-aryl-substituted dihydropyrimidine derivatives fused to pyrazole and triazole scaffolds via an efficient and green Domino reaction. Monatsh Chem 148, 1469–1475 (2017). https://doi.org/10.1007/s00706-017-1932-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-1932-x