Abstract
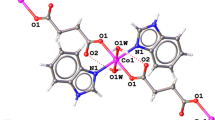
The reaction of aqueous cobaltous nitrate hexahydrate with the anion of succinimide (sucH) in the presence of excess ethylenediamine (en) in air results in the formation of a dinuclear complex µ-peroxo-bis[bis(ethylenediamine)succinimidato-cobalt(III)] dinitrate dihydrate, 1, in good yield. Compound 1 was characterized by elemental analysis, IR, visible spectra and magnetic susceptibility studies. The explosive nature of [Co(en)2(suc)(µ-O2)Co(en)2(suc)](NO3)2·2H2O, 1, precluded its thermal characterization. Compound 1 crystallises in the monoclinic space group P21/c and a half of the molecule, constitutes its asymmetric unit. In the centrosymmetric dinuclear complex 1, two Co(III) centres are linked by a planar peroxide bridge. Each cobalt atom is surrounded by four nitrogen atoms of ethylenediamine ligands, a nitrogen atom of succinimidato anion and an oxygen atom of peroxo bridge resulting in a slightly distorted {CoN5O} octahedron. Due to steric hindrance between the two Co(III) centres, the peroxide bridge is planar with a Co-O-O-Co torsion angle of 180°. The dinuclear complex cation, the nitrate anion and the lattice water are involved in three varieties of H-bonding interactions namely N-H⋯O, O-H⋯O and C-H⋯O.
Similar content being viewed by others
References
Ortego J D and Seymour M 1982 Polyhedron 1 21
Macarthur R, Sucheta A, Chong F F S and Einarsdottir O 1995 Proc. Natl. Acad. Sci. USA 92 8105
Martell E, Motekaitis R J, Rockcliffe D, Menif R and Ngwenya P M 1994 Pure and Appl. Chem. 66 859
Abakumov G A, Poddel’sky A I, Grunova E V, Cherkasov V K, Fukin G K, Kurskii Y A and Abakumova L G 2005 Angew. Chem. Int. Ed. 44 2767
Aires V V E, Zaccaron C M, Neves A and Szpoganicz B 2003 Inorg. Chim. Acta 353 82
Argay G, Fábián L and Kálmán A 1999 Croatica Chemica Acta 72 551
Yamada S and Miki S 1963 Bull. Chem. Soc. Jpn. 36 680
Slabbert N P and Thornton D A 1971 J. Inorg. Nucl. Chem. 33 2933
Akitsu T, Komorita S and Tamura H 2003 Inorg. Chim. Acta 348 25
Akitsu T and Komorita S 2002 Bull. Chem. Soc. Jpn. 75 767
Akitsu T, Komorita S and Kushi Y 2001 Inorg. Chim. Acta 315 18
Akitsu T, Komorita S and Kushi Y 1999 Bull. Chem. Soc. Jpn. 72 447
Salmain M, Jaouen G, Rudolf B and Zakrzewski J 1999 J. Organometallic. Chem. 589 98
Serrano J L, Zheng Y, Dilworth J R and Sánchez G 1999 Inorg. Chem. Commun. 2 407
Serrano J L, García L, Perez J, Perez E, Vives J, Sanchez G, Lopez G, Molins E and Orpen A G 2002 Polyhedron 21 1589
Akitsu T and Einaga Y 2005 Acta Cryst. C61 m183
Taş M, Saraçoğlu H, Batı H, Çalışkan N and Büyükgüngör O 2006 Z. Naturforsch. 61b 979
Jhon M S, Cho U, Kier L B and Eyring H 1972 Proc. Nat. Acad. Sci. USA 69 121
Stoe&Cie, X-Area (Version 1.18) and X-Red32 (Version 1.04), Stoe&Cie, Darmstadt, Germany, 2002
Burla M C, Caliandro R, Camalli M, Carrozzini B, Cascarano G L, De Caro L, Giacovazzo C, Polidori G and Spagna R SIR2004: A program for automatic solution and refinement of crystal structures 2005 J. Appl. Crystallogr. 38 381
Sheldrick G M 1997 SHELXL-97: Program for the refinement of crystal structures (Germany: University of Göttingen)
Johnson C K and Burnett M N 1997 ORTEP-III (version 1.0.2), Rep. ORNL-6895, Oak Ridge National Laboratory, Oak Ridge, TN (USA) (1996). Windows version: Farrugia L J, University of Glasgow, Glasgow, Scotland (UK)
Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Nakamoto K, Suzuki M, Ishiguro T, Kozuka M, Nishida Y and Kida S 1980 Inorg. Chem. 19 2822
Barraclough G, Lawrance G A and Lay P A 1978 Inorg. Chem. 17 3317
Shibahara T, Koda S and Mori M 1973 Bull. Chem. Soc. Jpn. 46 2070
McMullen S E and Hagen K S 2002 Acta Cryst. E58 m141
Fritch J R, Christoph G G and Schaefer W P 1973 Inorg. Chem. 12 2170
Etter M C 1990 Acc. Chem. Res. 23 120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taş, M., Büyükgüngör, O. Synthesis and structural characterization of a novel peroxo bridged dinuclear cobalt(III) complex of succinimide showing three varieties of hydrogen bonding interactions. J Chem Sci 121, 267–273 (2009). https://doi.org/10.1007/s12039-009-0029-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-009-0029-4