Abstract
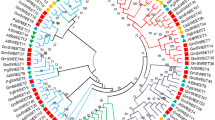
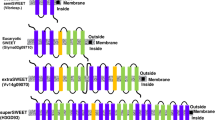
Sugar will eventually be exported transporters (SWEETs), a newly discovered class of sugar transporters, play a significant role in sugar efflux processes across various kingdoms of life. In fact, SWEETs have a long evolutionary path from prokaryotes to higher plants. In plants, they are involved in developmental processes, including nectar secretion, pollen nutrition, and seed filling. While the role of SWEETs has been well studied in biotic stresses, particularly their manipulation by pathogens for sugar acquisition, they have also been linked to many abiotic stresses. Although the phylogenetic relationships and solved structures of SWEETs in different plants have been revealed, their regulation remains unexplored. The current review deals with all the exciting discoveries around SWEETs, including their classification and diversity, and bridges the gaps in their evolutionary story, from bacterial semiSWEETs to eukaryotic SWEETs. We also critically examine SWEETs at genomic, transcriptomic, and proteomic levels, as evinced by recently published examples from grain, millet, and horticultural crops. In addition, we highlight the possibilities of utilizing SWEETs in applications such as bioethanol production and disease diagnostic markers. We attempt to elucidate and unify findings related to the yet unsolved puzzle of SWEET regulation in plants to improve crop production and protection for sustainable agriculture.



Similar content being viewed by others
References
Abelenda JA, Bergonzi S, Oortwijn M, Sonnewald S, Du M, Visser RG, Sonnewald U and Bachem CW 2019 Source-sink regulation is mediated by interaction of an FTHomolog with a SWEET protein in potato. Curr. Biol. 29 1178–1186
Abelenda JA, Navarro C and Prat S 2014 Flowering and tuberization: a tale of two nightshades. Trends Plant Sci. 19 115–122
An J, Zeng T, Ji C, de Graaf S, Zheng Z, Xiao TT, Deng X, Xiao S, Bisseling T, Limpens E and Pan Z 2019 AMedicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 224 396–408
Anjali A, Fatima U, Manu MS, Ramasamy S and Senthil-Kumar M 2020 Structure and regulation of SWEET transporters in plants: an update. Plant Physiol. Biochem. 156 1–6
Antony G, Zhou J, Huang S, Li T, Liu B, White F and Yang B 2010 Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 22 3864–3876
Ayre BG 2011 Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 4 377–394
Bezrutczyk M, Hartwig T, Horschman M, Char SN, Yang J, Yang B, Frommer WB and Sosso D 2018 Impaired phloem loading in zmsweet13a, b, c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 218 594–603
Bihmidine S, Baker RF, Hoffner C and Braun DM 2015 Sucrose accumulation in sweet sorghum stems occurs by apoplasmic phloem unloading and does not involve differential sucrose transporter expression. Bmcplant Biology 15 1–22
Bock KW, Honys D, Ward JM, et al. 2006 Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol. 140 1151–1168
Breia R, Conde A, Badim H, Fortes AM, Gerós H and Granell A 2021 Plant SWEETs: from sugar transport to plant–pathogen interaction and more unexpected physiological roles. Plant Physiol. 186 836–852
Bush DR 1993 Proton-coupled sugar and amino acid transporters in plants. Annu. Rev. Plant Biol. 44 513–542
Chardon F, Bedu M, Calenge F, et al. 2013 Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr. Biol. 23 697–702
Chen HY, Huh JH, Yu YC, et al. 2015a The Arabidopsis vacuolar sugar transporter SWEET 2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 83 1046–1058
Chen LQ 2014 SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 201 1150–1155
Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S and Fernie AR 2012 Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335 207–211
Chen LQ, Hou BH, Lalonde S, et al. 2010 Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468 527–532
Chen LQ, Lin IW, Qu XQ, Sosso D, McFarlane HE and Londoño A 2015b A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 27 607–619
Chong J, Piron MC, Meyer S, Merdinoglu D, Bertsch C and Mestre P 2014 The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 65 6589–6601
Chu Z, Fu B, Yang H, Xu C, Li Z and Sanchez A 2006 Targeting xa13 a recessive gene for bacterial blight resistance in rice. Theor. Appl. Genet. 112 455–461
Cohn M, Bart RS, Shybut M, Dahlbeck D and Gomez M 2014 Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector–mediated induction of a SWEET sugar transporter in cassava. Mol. Plant Microbe Interact. 27 1186–1198
Cox KL, Meng F, Wilkins KE, Li F, Wang P and Booher NJ 2017 TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 8 1–14
Crane E 1990 Bees and beekeeping: science practice and world resources, Heinemann Newnes
Davidson RM, Hansey CN, Gowda M, Childs KL, Lin H and Vaillancourt B 2011 Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 4
Desrut A, Moumen B, Thibault F, Le Hir R, Coutos-Thévenot P and Vriet C 2020 Beneficial rhizobacteria Pseudomonas simiae WCS417 induce major transcriptional changes in plant sugar transport. J. Exp. Bot. 71 7301–7315
Doucouré H, Pérez-Quintero AL, Reshetnyak G, Tekete C, Auguy F and Thomas E 2018 Functional and genome sequence-driven characterization of TAL effector gene repertoires reveals novel variants with altered specificities in closely related Malian Xanthomonas oryzae pv oryzae strains. Front. Microbiol. 9 1657
Durand M, Porcheron B, Hennion N, Maurousset L, Lemoine R and Pourtau N 2016 Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 170 1460–1479
Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW and Qu XQ 2015 SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 25 53–62
Eom JS, Luo D, Atienza-Grande G, Yang J, Ji C, Huguet-Tapia JC and Char SN 2019 Diagnostic kit for rice blight resistance. Nat. Biotechnol. 37 1372–1379
Etxeberria E, Pozueta-Romero J and Gonzalez P 2012 In and out of the plant storage vacuole. Plant Sci. 190 52–61
Fatima U and Senthil-Kumar M 2021 Sweet revenge: AtSWEET12 in plant defense against bacterial pathogens by apoplastic sucrose limitation. bioRxiv https://doi.org/10.1101/2021.10.04.463061v1
Feng CY, Han JX, Han XX and Jiang J 2015 Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene 573 261–272
Feng L and Frommer WB 2015 Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 40 480–486
Fernie AR, Bachem CW, Helariutta Y, Neuhaus HE, Prat S and Ruan YL 2020 Synchronization of developmental, molecular and metabolic aspects of source–sink interactions. Nat. Plants 6 55–66
Gamas P, de Carvalho Niebel F, Lescure N and Cullimore JV 1996 Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol. Plant Microbe. Interact. 9 233–242
Giaquinta RT 1983 Phloem loading of sucrose. Ann. Rev. Plant Physiol. 34 347–387
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX and Yang ZN 2008 RUPTUREDPOLLENGRAIN1 a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol. 147 852–863
Guo WJ, Nagy R, Chen HY, Pfrunder S, Yu YC and Santelia D 2014 SWEET17 a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 164 777–789
Gutjahr C and Parniske M 2013 Cell and developmental biology of arbuscular mycorrhiza symbiosis. Ann. Rev. Cell Dev. Biol. 29 593–617
Helber N, Wiel K, Sauer N, Schaarschmidt S, Hause B and Requena N 2011 A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell 23 3812–3823
Ho LH, Klemens PA, Neuhaus HE, Ko HY, Hsieh SY and Guo WJ 2019 SlSWEET1a is involved in glucose import to young leaves in tomato plants. J. Exp. Bot. 70 3241–3254
Hu YB, Sosso D, Qu XQ, Chen LQ, Ma L and Chermak D 2016 Phylogenetic evidence for a fusion of archaeal and bacterial SemiSWEETs to form eukaryotic SWEETs and identification of SWEET hexose transporters in the amphibian chytrid pathogen Batrachochytrium dendrobatidis. FASEB J. 30 3644–3654
Jaehme M, Guskov A and Slotboom DJ 2014 Crystal structure of the vitamin B 3 transporter PnuC, a full-length SWEET homolog. Nat. Struct. Mol. Biol. 21 1013–1015
Jiang Y, Wang W, Xie Q, et al. 2017 Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356 1172–1175
Jiang L, Song C, Zhu X and Yang J 2021 SWEET transporters and the potential functions of these sequences in tea (Camellia sinensis). Front. Genet.
Jung B, Ludewig F, Schulz A, et al. 2015 Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat. Plants 1 1–6
Kanno Y, Oikawa T, Chiba Y, et al. 2016 AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat. Commun. 7 1–11
Ke Y, Hui S and Yuan M 2017 Xanthomonas oryzae pv oryzae inoculation and growth rate on rice by leaf clipping method. Bio-Protocol 7 e2568
Kiers ET, Duhamel M, Beesetty Y, et al. 2011 Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333 880–882
Kim P, Xue CY, Song HD, Gao Y, Feng L and Li Y 2021 Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 19 409
Klemens PA, Patzke K, Deitmer J, et al. 2013 Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 163 1338–1352
Ko HY, Ho LH, Neuhaus HE and Guo WJ 2021 Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato. Plant Physiol.
Kryvoruchko IS, Sinharoy S, Torres-Jerez I, et al. 2016 MtSWEET11 a nodule-specific sucrose transporter of Medicago truncatula. Plant Physiol. 171 554–565
Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V and Meena RC 2008 Effect of waterlogging on carbohydrate metabolism in pigeon pea (Cajanus cajan L): upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci. 175 706–716
Lee Y, Nishizawa T, Yamashita K, Ishitani R and Nureki O 2015 Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter. Nat. Commun. 6 1–8
Li T, Liu B, Spalding MH, Weeks DP and Yang B 2012 High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 30 390–392
Li Y, Liu H, Yao X, et al. 2021 Hexose transporter CsSWEET7a in cucumber mediates phloem unloading in companion cells for fruit development. Plant Physiol. 186 640–654
Lin IW, Sosso D, Chen LQ, et al. 2014 Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 508 546–549
Liu HT, Lyu WY, Tian SH, Zou XH, Zhang LQ, Gao QH, Ni DA and Duan K 2019 The SWEET family genes in strawberry: identification and expression profiling during fruit development. South African J. Bot. 125 176–187
Ludewig U, Wilken S, Wu B, et al. 2003 Homo-and hetero-oligomerization of ammonium transporter-1 NH 4+ uniporters. J. Biol. Chem. 278 45603–45610
Ma L, Zhang D, Miao Q, Yang J, Xuan Y and Hu Y 2017 Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol. 58 863–873
Mackill DJ and Khush GS 2018 IR64: a high-quality and high-yielding mega variety. Rice 11 1–11
Malinowski R, Truman W and Blicharz S 2019 Genius architect or clever thief—how Plasmodiophora brassicae reprograms host development to establish a pathogen-oriented physiological sink. Mol. Plant Microbe Interact. 32 1259–1266
Manck-Götzenberger J and Requena N 2016 Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 7 487
Martinoia E, Meyer S, De Angeli A and Nagy R 2012 Vacuolar transporters in their physiological context. Annu. Rev. Plant Biol. 63 183–213
Mathan J, Singh A and Ranjan A 2021a Sucrose transport and metabolism control carbon partitioning between stem and grain in rice. J. Exp. Bot. 72 4355–4372
Mathan J, Singh A and Ranjan A 2021b Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 171 620–637
McGaughey SA, Osborn HL, Chen L, Pegler JL, Tyerman SD and Furbank RT 2016 Roles of aquaporins in Setaria viridis stem development and sugar storage. Front. Plant Sci. 7 1815
Mewis I, Khan MA, Glawischnig E, Schreiner M and Ulrichs C 2012 Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana. PLoS One 7 e48661
Miao H, Sun P, Liu Q, et al. 2017 Genome-wide analyses of SWEET family proteins reveal involvement in fruit development and abiotic/biotic stress responses in banana. Sci. Rep. 7 1–15
Mizuno H, Kasuga S and Kawahigashi H 2016 The sorghum SWEET gene family: stem sucrose accumulation as revealed through transcriptome profiling. Biotechnol. Biofuels 9 1–12
Morii M, Sugihara A, Takehara S, et al. 2020 The dual function of OsSWEET3a as a gibberellin and glucose transporter is important for young shoot development in rice. Plant Cell Physiol. 61 1935–1945
Navarro C, Abelenda JA, Cruz-Oró E, Cuellar CA, Tamaki S, Silva J, Shimamoto K and Prat S 2011 Control of flowering and storage organ formation in potato by FLOWERINGLOCUST. Nature 478 119–122
Navarro C, Cruz-Oró E and Prat S 2015 Conserved function of FLOWERINGLOCUST (FT) homologues as signals for storage organ differentiation. Curr. Opin. Plant Biol. 23 45–53
NIÑO-LIUDO, Ronald PC and Bogdanove AJ, 2006 Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7 303–324
Oliva R, Ji C, Atienza-Grande G, et al. 2019 Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 37 1344–1350
Phukan UJ, Jeena GS, Tripathi V and Shukla RK 2018 Ma RAP 2–4 a waterlogging-responsive ERF from Mentha, regulates bidirectional sugar transporter At SWEET 10 to modulate stress response in Arabidopsis. Plant Biotechnol. J. 16 221–233
Riesmeier JW, Willmitzer L and Frommer WB 1992 Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11 4705–4713
Roy R, Schmitt AJ, Thomas JB and Carter CJ 2017 Nectar biology: from molecules to ecosystems. Plant Sci. 262 148–164
Salts Y, Sobolev I, Chmelnitsky I, Shabtai S and Barg R 2005 Genomic structure and expression of Lestd1 a seven-transmembrane-domain proteon-encoding gene specially expressed in tomato pollen. Israel J. Plant Sci. 53 79–88
Sarkar RK and Ray A 2016 Submergence-tolerant rice withstands complete submergence even in saline water: Probing through chlorophyll a fluorescence induction OJIP transients. Photosynthetica 54 275–287
Sarkar RK, Das KK, Panda D, Reddy JN, Patnaik SS C, Patra BC and Singh DP 2014 Submergence tolerance in rice: biophysical constraints, physiological basis and identification of donors (Central Rice Research Institute, Cuttack, India) p36
Sauer N and Stolz J 1994 SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. Plant J. 6 67–77
Schulz A, Beyhl D, Marten I, et al. 2011 Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 68 129–136
Schulze WX, Reinders A, Ward J, Lalonde S and Frommer WB 2003 Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem. 4 1–10
Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR and De Leon N 2011 Genome-wide atlas of transcription during maize development. Plant J. 66 553–563
Seo PJ, Park JM, Kang SK, Kim SG and Park CM 2011 An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 233 189–200
Siemen J, González MC, Wolf S, et al. 2011 Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Pathol. 12 247–262
Singh J, James D, Achary VM M, Patel MK, Thakur JK, Reddy MK and Tripathy BC 2021 Coordinated overexpression of OsSUT1 OsSWEET11 and OsSWEET14 in rice impairs carbohydrate metabolism that has implications in plant growth, yield and susceptibility to Xanthomonas oryzae pv oryzae (Xoo). bioRxiv https://doi.org/10.1101/2021.01.07.425507
Smith FA and Smith SE 2011 What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant Soil 348 63–79
Song WY, Wang GL, Chen LL, et al. 1995 A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270 1804–1806
Sosso D, Luo D, Li QB, et al. 2015 Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 47 1489–1493
Streubel J, Pesce C, Hutin M, Koebnik R, Boch J and Szurek B 2013 Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv oryzae. New Phytol. 200 808–819
Sun MX, Huang XY, Yang J, Guan YF and Yang ZN 2013 Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod. 26 83–91
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S and Zhang Q 2004 Xa26 a gene conferring resistance to Xanthomonas oryzae pv oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 37 517–527
Takanaga H and Frommer WB 2010 Facilitative plasma membrane transporters function during ER transit. FASEB J. 24 2849–2858
Tao Y, Cheung LS, Li S, Eom JS, Chen LQ and Xu Y 2015 Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 527 259–263
Toledo AMU, Ignacio JCI, Casal C, Gonzaga ZJ, Mendioro MS and Septiningsih EM 2015 Development of improved Ciherang-Sub1 having tolerance to anaerobic germination conditions. Plant Breed. Biotechnol. 2015 77–87
Tran TT, Pérez-Quintero AL, Wonni I, et al. 2018 Functional analysis of African Xanthomonas oryzae pv oryzae TALomes reveals a new susceptibility gene in bacterial leaf blight of rice. PLoS Pathogen. 14 e1007092
Vacheron J, Desbrosses G, Bouffaud ML, et al. 2013 Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4 356
Valifard M, Le Hir R, Müller J, Scheuring D, Neuhaus HE and Pommerrenig B 2021 Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance. Plant Physiol
Walerowski P, Gündel A, Yahaya N, et al. 2018 Clubroot disease stimulates early steps of phloem differentiation and recruits SWEET sucrose transporters within developing galls. Plant Cell 30 3058–3073
Wang H, Yan S, Xin H, et al. 2019a A subsidiary cell-localized glucose transporter promotes stomatal conductance and photosynthesis. Plant Cell 31 1328–1343
Wang L, Yao L, Hao X, et al. 2018 Tea plant SWEET transporters: expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 96 577–592
Wang S, Liu S, Wang J, et al. 2020 Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Nat. Sci. Rev. 7 1776–1786
Wang S, Yokosho K, Guo R, Whelan J, Ruan YL, Ma JF and Shou H 2019b The soybean sugar transporter GmSWEET15 mediates sucrose export from endosperm to early embryo. Plant Physiol. 180 2133–2141
Wu Y, Lee SK, Yoo Y, Wei J, Kwon SY, Lee SW, Jeon JS and An G 2018 Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET. genes. Mol. Plant 11 833–845
Xiang Y, Cao Y, Xu C, Li X and Wang S 2006 Xa3 conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor. Appl. Genet. 113 1347–1355
Xiong Y, Li QB, Kang BH and Chourey PS 2011 Discovery of genes expressed in basal endosperm transfer cells in maize using 454 transcriptome sequencing. Plant Mol. Biol. Rep. 29 835–847
Xu Y, Tao Y, Cheung LS, et al. 2014 Structures of bacterial homologues of SWEET transporters in two distinct conformations. Nature 515 448–452
Xuan YH, Hu Y 0B, Chen LQ, Sosso D, Ducat D, Hou BH and Frommer WB 2013 Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Nat. Acad. Sci. 110 E3685–E3694
Yang B and White FF 2004 Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant Microbe Interact. 17 1192–1200
Yang B, Sugio A and White FF 2006 Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Nat. Acad. Sci. 103 10503–10508
Yang J, Luo D, Yang B, Frommer WB and Eom JS 2018 SWEET 11 and 15 as key players in seed filling in rice. New Phytol. 218 604–615
Yao L, Ding C, Hao X, Zeng J, Yang Y, Wang X and Wang L 2020 CsSWEET1a and CsSWEET17 mediate growth and freezing tolerance by promoting sugar transport across the plasma membrane. Plant Cell Physiol. 61 1669–1682
Yu Y, Streubel J, Balzergue S, et al. 2011 Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv oryzae depends on a new TAL effector that induces the rice nodulin-3 Os11N3 gene. Mol. Plant Microbe Interact. 24 1102–1113
Yuan M, Chu Z, Li X, Xu C and Wang S 2010 The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22 3164–3176
Zhang Z, Zou L, Ren C, Ren F, Wang Y, Fan P, Li S and Liang Z 2019 VvSWEET10 mediates sugar accumulation in grapes. Genes 10 255
Zhen Q, Fang T, Peng Q, Liao L, Zhao L, Owiti A and Han Y 2018 Developing gene-tagged molecular markers for evaluation of genetic association of ale SWEET genes with fruit sugar accumulation. Hort. Res. 5 1–12
Zhou J, Peng Z, Long J, et al. 2015 Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 82 632–643
Acknowledgements
The SWEET transporter project at MS-K’s lab was funded by NIPGR core funding. UF acknowledges the DBT-SRF fellowship (DBT/2013/NIPGR/68) and NIPGR-SRF fellowship. AA acknowledges the CSIR fellowship (09/803(0168)/2019-EMR-I_367532).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Manchikatla Venkat Rajam.
Corresponding editor: Manchikatla Venkat Rajam
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anjali, A., Fatima, U. & Senthil-Kumar, M. The ins and outs of SWEETs in plants: Current understanding of the basics and their prospects in crop improvement. J Biosci 46, 100 (2021). https://doi.org/10.1007/s12038-021-00227-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-021-00227-6