Abstract
DNA damage is associated with hyperhomocysteinemia (HHcy) and neural tube defects (NTDs). Additionally, HHcy is a risk factor for NTDs. Therefore, this study examined whether DNA damage is involved in HHcy-induced NTDs and investigated the underlying pathological mechanisms involved. Embryonic day 9 (E9) mouse neuroectoderm cells (NE4C) and homocysteine-thiolactone (HTL, active metabolite of Hcy)-induced NTD chicken embryos were studied by Western blotting, immunofluorescence. RNA interference or gene overexpression techniques were employed to investigate the impact of Menin expression changes on the DNA damage. Chromatin immunoprecipitation-quantitative polymerase chain reaction was used to investigate the epigenetic regulation of histone modifications. An increase in γH2AX (a DNA damage indicator) was detected in HTL-induced NTD chicken embryos and HTL-treated NE4C, accompanied by dysregulation of phospho-Atr-Chk1-nucleotide excision repair (NER) pathway. Further investigation, based on previous research, revealed that disruption of NER was subject to the epigenetic regulation of low-expressed Menin-H3K4me3. Overexpression of Menin or supplementation with folic acid in HTL-treated NE4C reversed the adverse effects caused by high HTL. Additionally, by overexpressing the Mars gene, we tentatively propose a mechanism whereby HTL regulates Menin expression through H3K79hcy, which subsequently influences H3K4me3 modifications, reflecting an interaction between histone modifications. Finally, in 10 human fetal NTDs with HHcy, we detected a decrease in the expression of Menin-H3K4me3 and disorder in the NER pathway, which to some extent validated our proposed mechanism. The present study demonstrated that the decreased expression of Menin in high HTL downregulated H3K4me3 modifications, further weakening the Atr-Chk1-NER pathway, resulting in the occurrence of NTDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated plasma concentrations of total homocysteine are defined as hyperhomocysteinemia (HHcy) [1], which has been linked to the induction of neural tube defects (NTDs) [2]. HHcy is caused by a maternal suboptimal intake of the nutrient folate, leading to the dysregulated activities of methionine and folate metabolism [2, 3]. NTDs can be mitigated by reducing total homocysteine levels through folic acid (FA) supplementation [1]. However, the precise mechanisms underlying HHcy-induced NTDs and the protective effects exerted by FA remain poorly understood.
The pathology of NTDs is linked to elevated reactive oxygen species (ROS) levels [4], DNA damage [5, 6], and cell-cycle arrest [7]. These effects parallel those of HHcy, which increases ROS levels [8, 9], promotes DNA damage [10], and induces G1 cell-cycle arrest [11]. Additionally, impaired DNA damage repair and genomic instability are common in NTDs and HHcy [12,13,14]. To maintain genome integrity under normal conditions, the ataxia-telangiectasia mutated (ATM)-checkpoint kinase 2 (Chk2) DNA damage response (DDR) pathway is activated by DNA double-strand breaks, and the ataxia-telangiectasia and rad3-related protein (ATR)-checkpoint kinase 1 (Chk1) DDR pathway can be activated by nucleotide excision and DNA mismatch [15]. The activated ATR-Chk1 pathway triggers a complex signaling network, which plays a crucial role in checkpoint control during DNA damage, and causes cell cycle pause to allow sufficient time for nucleotide excision repair (NER) [16]. Specifically, this pathway can directly drive NER by phosphorylating and activating xeroderma pigmentosum group A (XPA). Subsequently, the XPA that recognizes the damaged sites interacts with other components of the NER pathway such as excision repair 8 (Ercc8), DNA damage binding protein 1 (Ddb1), cullin 4A (Cul4a), ubiquitin specific peptidase 7 (Usp7), and DNA ligase 1 (Lig1), to facilitate the repair of DNA damage [17,18,19,20]. Wang et al. discovered that HHcy impairs ATR, causing cell-cycle checkpoint and DDR dysregulation, thereby suppressing DNA damage repair [14, 21]. Collectively, these findings suggest that HHcy may induce NTDs through the dysregulation of DDR.
A potential mechanism of HHcy toxicity involves the non-enzymatic binding of homocysteine thiolactone (HTL) (a highly reactive hcy metabolite is generated by methionyl-tRNA synthetases (MARS)) to intracellular and extracellular proteins during the post-translational process. This forms protein lysine (N)-homocysteinylation modifications, which impair protein function and disrupt hcy metabolism, thereby exerting adverse effects on cellular function and embryonic development [22,23,24,25,26]. In a FA deficiency-induced NTD mouse model, maternal serum folate levels had a significant negative correlation with serum hcy levels, whereas serum hcy levels had a significant positive correlation with the titer of autoantibodies against homocysteinylated proteins [25], suggesting that a low-folate diet inducing NTDs might increase hcy and protein homocysteinylation. HHcy promotes the N-homocysteinylation of superoxide dismutase-1/2 (SOD1/2) leading to the inactivation of SOD1/2, thereby increasing ROS levels [13]. This provides a new explanation for the oxidative stress induced by HHcy during the development of NTDs. Additionally, histone homocysteinylation has a critical role in the pathogenesis of HHcy-induced NTDs. As demonstrated in our previous study, HTL can elevate H3K79 homocysteinylation levels (H3K79hcy) in a dose-dependent manner in NE4C cells, which further epigenetic regulation of the expression of neural tube closure-related genes such as Smarca4, Cecr2, and Dnmt3b [23]. Our research outcomes have raised a captivating question: Does HTL mediate histone homocysteinylation modifications, thereby triggering an imbalance in DDR? In addition, we also observed that high HTL could potentially impact the methylation of histones [23]. Considering histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 79 dimethylation (H3K79me2) can epigenetically regulate the transcription of key genes involved in neural tube closure [28, 29], we further propose this question: Does a cooperative effect exist between histone homocysteinylation and methylation modifications in the process of DDR disruption caused by HHcy or high HTL? If so, what is the specific mechanism behind it?
Here, we established HTL-treated NE4C cells and an HTL-induced NTDs chicken embryo model and collected HHcy-related human fetal NTD samples to examine their DNA damage status. By generating Men1-overexpressing (Men1-OE) NE4C cells, we further explored the epigenetic regulatory mechanisms that mediate genomic stability to elucidate the pathogenesis of high HTL-induced NTDs.
Methods
Human Subjects
Ten cases of HHcy-related NTDs and 10 normal control samples were obtained from Lvliang, Shanxi, China. NTDs were diagnosed according to the International Classification of Diseases 10th edition code, with early pregnancy ultrasound screening. The specific phenotypes of NTDs were mostly spina bifida malformations; the main clinical characteristic was incomplete vertebral canal closure in the lower spinal region, resulting in a split spine. Six of which were accompanied by hydrocephalus. Control fetuses matched with HHcy-related NTD group were miscarried due to non-medical reasons (voluntary early abortion for non-health or non-pathological reasons), excluding malformations or fetal intrauterine growth retardation. And the controls and the NTD group were matched in terms of the mother’s age and the gender of the fetus. Both groups of mothers did not take FA supplements (Supplementary Table 1) [23]. We extracted the brain tissue of NTDs and control fetuses for subsequent measurement.
Hcy Level Detection
Twenty milligrams of fetal brain tissue was treated with 150 mL of 50 mM dithiothreitol for 20 min to reduce disulfide bonds, followed by the addition of 200 µL internal standard (Hcy-d4). After sonication and centrifugation at 12,000 g, the supernatant was transferred to SPE tips. Hcy was dissolved in methanol–water (65:35, v/v) containing 1 mM ammonium formate. An Agilent 6410B mass spectrometer was coupled to an Agilent 1200 system HPLC for sample separation using a Zorbax Bonus-RP column with a flow rate of 0.25 mL/min. The column temperature was set to 35 °C; MS/MS experiments were conducted in ESI + mode, performing MRM monitoring. MRM transitions for Hcy and Hcy-d4 were 350–204.1 and 354.2–208.1, respectively [23].
HTL Treatment of Chicken Embryos
The samples required for this study originate from chicken embryo samples used in previous studies [23, 27]. The simplified steps for HTL induction of chicken embryos are as follows: Translate the fertile white leghorn chicken eggs (obtained from the China Agricultural University) [28] into a 37 ℃ humidified incubator and incubate for 28–30 h (i.e., E1-E2 stage). Then, inject 0.5 µl of 0.5 mM HTL solution into the neural groove under a dissection microscope and seal the egg tightly. Continue to incubate until the E2, E4, E5, or E8 stages and observe the chick embryo phenotype. The 0.5 mM HTL solution was obtained by diluting HTL (H6503, Sigma) in Tyrode’s buffer (T1420, Solarbio, China), which includes 134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, and 20 mM Hepes, and phenol red was added to facilitate observation of the embryo’s condition [29]. The control group was injected with an equivalent volume of Tyrode’s buffer and phenol red.
NE4C and SV129 Cell Culture and HTL Treatment
E9 mouse neural stem cells (NE4C) (SCRC-CRL-2925™) were cultured in pre-coated poly L-lysine T25 plates with passaging medium (90% MEM (11,090,073, Thermofisher); 10% FBS (10099141C, Thermofisher)) [23]. E4.5 SV129 cells were provided by Xuanwu Hospital, pre-coat gelatin plates, growth medium: 85% DMEM, etc. [29]. Cells are cultured in a 37 °C humidified environment with 5% CO2, and the passage ratio is 1:4 to 1:7. After starvation treatment (using 1% FBS medium for 24 h), cells are incubated in FBS medium containing 0.5/1 mM HTL for 8 h, while untreated cells serve as controls.
Men1/Mars Overexpression in NE4C
Mouse Men1 cDNA (NM_001168488) (containing 1854 bp CDS) was cloned into a pcDNA3.1 vector (purchased from Sangon Biotech, China); And mouse Mars cDNA (NM_004990) was cloned into a pRK7 vector, with 2700 bp ORF size (Sangon Biotech) [13]. The cells were initially cultured until they reached 30–40% confluence in a medium free from antibiotics before undergoing transfection with Men1- and Mars-specific plasmids by Lipofectamine transfection reagent (L3000075, Thermofisher), all carried out following the guidelines provided by the manufacturer. Concurrently, an empty vector was employed as a control measure. Post incubation for a period spanning 24 to 96 h, the cells were collected for use in subsequent experiments. The grouping information is as follows: cells transfected empty vector as “NC” group, cells transfected Men1 cDNA as “Men1 ( +)” group, cells transfected Mars cDNA as “Mars ( +)” group.
Western Blotting (WB)
Histones were extracted by acid extraction method from the tissues or cells [23, 30]. Total proteins were extracted by using the Protein Extraction Kit (C006225, Sango Biotech). Ten-microgram protein samples per lane were separated by 4–12% NuPAGE™ Bis–Tris gel (NP032B, Thermo Scientific™). Primary antibodies used were as follows: DNA Damage Antibody Sampler Kit (#9947, Cell Signaling Technology (CST)), including phospho-ATR (Ser428) (#2853, CST), phospho-ATM (Ser1981) (#5883, CST), phospho-Chk2 (Thr68) (#2197, CST), phospho-Chk1 (Ser345) (#2348, CST), phospho-Histone H2A.X (Ser139) (#9718, CST), ATR (#13,934, CST), and CHK1 (#2360, CST), and SET1/COMPASS Antibody Sampler Kit (#25,501, CST), including Mll1 (#14,689, CST), Menin (#6891, CST), and Wdr82 (#99,715, CST); MARS (ab180497) was purchased from Abcam. In addition, we also used H3K4me3 (#9751, CST), H3K79hcy [23], H3K79me2 (#5427, CST), H2AX (#7631, CST), and H3 (Ab267372, Abcam) for detection of histone modifications. The intensity of blots was quantified with densitometry using by using an Image Lab software (Gel Image System, Tanon, Shaihai, China).
Immunofluorescence (IF)
Cells were firstly washed with 1 × PBS twice and treated as follows: fixation with 0.5% paraformaldehyde for 15 min and then permeabilized in 0.5% Triton X-100 for 20 min at room temperature and blocked with 1 × PBS containing 10% normal goat serum and 0.3 M glycine for 60 min. Cells were incubated with γ-H2A.X antibody diluted in 5% normal goat serum overnight at 4 °C. After washing, secondary antibodies with Alexa Fluor 488 (ab150077, Abcam) were used for 1-h incubation at room temperature in the dark. Nuclei were counterstained with DAPI. Images were captured on a Zeiss LSM710 confocal microscope.
ChIP-qPCR
According to the manufacturer’s protocol, SimpleChIP® Enzymatic Chromatin IP Kit (#9002, CST) was used for the ChIP assays. Formaldehyde cross-linked chromatin was obtained from about 1 × 107 cells and further immunoprecipitated with H3K79Hcy/H3K4me3 antibodies overnight at 4 °C. Normal rabbit IgG was used as a negative control. DNA–protein complexes were analyzed by qPCR and primers were designed by Primer 3 in gene promoter regions (Primers used in this experiment are listed in Supplementary Table 2). Relative enrichment of H3K79hcy/H3K4me3 on genes was determined using the 2(C[T] input sample ‑C[T] IP sample)Case/2(C[T] input sample ‑C[T] IP sample)Control method, C[T] = Ct = threshold cycle of the PCR reaction [29].
Transcription Analysis by RT-qPCR
The TRIzol reagents (15,596,026, Invitrogen) were used for extraction of total RNA from fresh-frozen tissues or fresh-harvested cells. Then, RNA was reverse transcribed using a First Strand cDNA Synthesis kit (K1612, TransGen Biotech). The qPCRs were performed on an Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific, Inc.) using PCR UltraSYBR Mixture kit (CW0956, CWBIO). The expression levels of the target genes were normalized to Gapdh expression levels, and the data was calculated according to the cycle threshold (2−△△Ct) method. The primers are listed in Supplementary Table 3.
Nanostring for mRNA Detection
Total RNA was extracted from E5 chicken embryo brain tissue sample using the RNeasy Plus Kit (Qiagen, Canada). The NanoString nCounter System detection method (NanoString Technologies) was used to examine the transcription levels of genes. Hybridization was performed according to the NanoString Gene Expression Assay manual [31]. Briefly, each target mRNA was made into a unique multiplex probe using two sequence-specific probes. The two probes complementarily construct in a target region of 100 bases. The capture probe consists of a target-specific oligonucleotide and biotin. The reporting probe was composed of the second target-specific oligonucleotide, connected to a unique dye-labeled RNA fragment chain for system detection. Each RNA sample of about 100 ng was mixed with 20 µL of nCounter reporter probes and 5 µL of nCounter capture probes in the hybridization buffer. Purified target/probe complexes were further eluted and immobilized on a cartridge for data collection, which is carried out in the nCounter digital analyzer. Results were normalized against GAPDH, CLTC, and GUSB genes.
Statistical Analysis
The SPSS software version 25.0 (SPSS Co., Chicago, IL) was used for statistical analysis. The Shapiro–Wilk test was used to assess the normality of all continuous variables. For the normality data (PCR and Western blot data in this study), data were expressed as means ± SEM, and unpaired Student’s two-tailed t test was used when comparing two groups; Comparisons among multiple groups were examined by one-way ANOVA plus post-hoc test. For the nonnormal distribution data, they were expressed as medians (interquartile ranges) and were analyzed via the Wilcoxon Mann–Whitney test (two groups) and the Kruskal–Wallis non-parametric test with post-hoc Bonferroni correction for multiple comparisons. For categorical variables, χ2 test or Fisher exact probability test was used for the comparisons between groups. Histograms were mainly drew by GraphPad Prism 8.0 (GraphPad Software, USA) and differences were considered statistically significant if the P value was less than 0.05.
Results
High HTL Induces DNA Damage Accumulation and Inactivates the Atr-Chk1-NER Signaling Pathway
We previously established a chicken embryo NTD model via HTL induction; specifically, we studied 107 chicken embryos treated with HTL and found that at E2 (48 h after embryo incubation), no stillbirths were observed, and the rate of embryonic malformations was 59.1%; at E3 (72 h after embryo incubation), the stillbirth rate was 9.09%, and the rate of embryonic malformations was 50%; at E5 (120 h after embryos incubation), the stillbirth rate was 15.1%, and the rate of embryonic malformations was 56.6%; at E8, the stillbirth rate was 10%, and the rate of embryonic malformations was 50%. These embryonic deformities included NTDs, heart defects, cerebral atrophy, and tail deformities. Particularly, the incidence of NTDs was about 36%, of which spina bifida accounted for about 10%, while brain herniation and other brain malformations constituted about 26% [23, 27]. Figure 1A shows typical spinal cord closure deformities at stage E2 and brain developmental malformations at stages E4 and E5. We observed an increase in γH2AX levels in the brain tissues of NTD embryos at stages E4 and E5 induced by high HTL (belong to the 36% of embryos with NTDs) (Fig. 1B), suggesting the occurrence of DNA damage in high HTL-induced NTDs. Moreover, our findings indicated a gradual increase in DNA damage from E3 to E5, implying that DNA damage may have a role in the progression of NTDs. Additionally, we also observed a decrease in the mRNA expression levels of key genes in the DDR pathway, such as Atr, Chek1, Xpa, Ercc8, and Cul4a, in the brain tissues of E5 chicken embryos after HTL treatment, while the expression levels of Atm, Chek2, Ddb1, Usp7, and Lig1 remained unchanged (Supplementary Fig. 1A). These results indicate that high HTL may lead to DNA damage in vivo and disrupt the pathway of DDR.
Increase of DNA damage in HTL-treated chicken embryos and NE4C. A Eggs treated with 0.5 mM-HTL exhibited spinal bifida aperta at the stage of embryo 2 (E2) (arrow indicated) and neural tube deformities at the stage of E4 and E5. B Increase in γH2A.X expression during brain development in high-HTL-treated chicken (n = 3). E1, 24 h after embryo incubation; E2, 48 h after embryo incubation; E3, 72 h after embryo incubation; E4, 96 h after embryo incubation; E5, 120 h after embryo incubation. The P values were calculated with unpaired t test. C The expression of γH2A.X in NE4C cells treated with 1 mM HTL was significantly increased compared to those treated with 0 mM or 0.5 mM HTL (n = 3). The P values were calculated with one-way ANOVA plus post-hoc test. D Left panel: IF method confirmed enhanced γH2A.X in 1 mM HTL treated NE4C cells; right panel: quantification of the IF signal intensity shown in scatter plot (n = 3). The P values were calculated with unpaired t test. E Decreased RNA content of Atr, Atm, and Chek2 in HTL-treated NE4C cells by RNA-seq analysis. F RT-qPCR validated decreased mRNA expression level of Atr, Atm, Chek1, and Xpa in 1 mM HTL-treated NE4C cells (n = 3). The P values were calculated with unpaired t test. G–H Reduced protein expression of p-Atr, p-Chk1, and Xpa in 1 mM HTL-treated NE4C cells compared to 0/0.5 mM HTL-treated cells by WB analysis (n = 3). The P values were calculated with one-way ANOVA plus post-hoc test
We further assessed DNA damage in NE4C cells using WB and IF. An increase in DNA damage was observed in cells treated with 1 mM HTL (Fig. 1C–D). DDR pathway-related genes (Atr, Atm, Chek2) were suppressed, as confirmed by RNA-seq [23] and RT-qPCR (Fig. 1E–F). Additionally, phospho-Atr, phospho-Atm, and phospho-Chk1 protein expressions were reduced in the 1 mM HTL-treated group, whereas phospho-Chk2 remained unchanged (Fig. 1G–H). We also found that the initiator of the NER pathway, Xpa, and its downstream genes Ercc8, Ddb1, Cul4a, Usp7, and Lig1 was significantly downregulated in 1 mM HTL-treated NE4C and SV129 cells (Supplementary Fig. 1B). These findings indicate that high HTL-induced DNA damage in neural stem cells inhibited the DNA damage response Atr-Chk1 pathway (rather than the Atm-Chk2 pathway), leading to an impaired Xpa-driven NER pathway.
Downregulated Menin-H3K4me3 is an Effector in the High HTL-Induced DNA Damage Response
To investigate the molecular mechanisms of high HTL involved in inhibiting DDR pathways, we reviewed our previous research and found that histone methylation levels change under high HTL conditions. Furthermore, 18 out of 45 genes, including Ercc8, Ddb1, Clu4a, and Usp7, in the NER pathway were regulated by H3K4me3 (Fig. 2A–B; Supplementary Table 4–5) [23]. Concurrently, other studies reported that the H3K4me3 methyltransferases Menin and MLL1 regulated DNA damage repair [32, 33]. Taken together, we hypothesize that MLL1/Menin-regulated H3K4me3 has a crucial role in the inhibition of DDR by high HTL. Consequently, we examined the modification level of H3K4me3 and the protein expression levels of related methyltransferases.
Decreased of Menin regulates declined H3K4me3 level in HTL-treated NE4C cells. A The top 30 enriched pathways of H3K4me3 dysregulated genes were analyzed by KEGG. The term “rich factor” refers to the ratio of the number of differentially expressed genes located in a particular pathway entry to the total number of annotated genes located in that pathway entry. B H3K4me3 ChIP-seq analysis on NER genes: Xpa, Ercc8, Ddb1, and Cul4a in 0 and 1 mM HTL-treated NE4C cells. C–D WB analysis demonstrated decreased Menin protein expression and H3K4me3 modification in 1 mM HTL-treated NE4C cells (n = 3). The P values were calculated with one-way ANOVA plus post-hoc test. E–F Overexpressed of Men1 in 0 mM and 1 mM HTL-cultured NE4C, respectively, which further induced an increased of H3K4me3 (n = 3). The symbol “-” indicates that the cell was not transfected with any plasmid; “NC” denotes that the cell was transfected with an empty plasmid; “ + ” denotes that the cell was transfected with a plasmid overexpressing the Men1 gene. The P values were calculated with one-way ANOVA plus post-hoc test
WB results indicated that Menin protein expression levels were significantly downregulated in 1 mM HTL-treated NE4C cells (also significantly downregulated in chicken embryonic brain tissue induced by HTL at E5 stage), whereas Mll1 and Wdr82 (other H3K4me3 methyltransferases) remained unchanged (Fig. 2C–D; Figure S2A). This accompanied a decrease in H3K4me3 modification levels (H3K79me2, a control, showed no difference). These data suggest that downregulated Menin protein and H3K4me3 modification levels might be effectors of high HTL. However, it will be necessary to confirm whether H3K4me3 is regulated by Menin under high HTL conditions.
Therefore, we overexpressed the Men1 gene in normally cultured cells and 1 mM HTL-treated cells (Fig. 2E–F). The results showed that Menin overexpression led to an increase in H3K4me3 modification levels (with no impact on H3K79me2), suggesting that Menin regulates H3K4me3 modification levels under normal and high HTL conditions. Menin protein may be a precursor effector molecule through which high HTL epigenetically regulates DDR pathways via H3K4me3.
A Decrease in Menin-H3K4me3 Regulates the Dysregulation of the Atr-Chk1-NER Pathway in High HTL
To determine the impact of the downregulation of the Menin-H3K4me3 cascade on DDR pathway activity within an HHcy environment, we overexpressed Men1/Menin in normal and 1 mM HTL-treated cell groups. Our findings revealed that within the HTL group, Menin overexpression significantly diminished γH2AX levels, thereby reducing DNA damage. Moreover, the expression level and activity of ATR increased with the elevation of Menin protein expression, even under an HHcy environment. P-CHK1 and XPA exhibited similar expression trends, suggesting the impaired DDR pathways under high HTL conditions might be ameliorated by Menin (Fig. 3A–B). Subsequently, we investigated whether the influence of Menin on the DDR pathway was mediated by H3K4me3 epigenetic regulation. Utilizing ChIP-qPCR technology, we observed that, in comparison to the “HTL (0 mM) + Men1-NC” group, the binding levels of H3K4me3 at the promoter regions of genes including Xpa, Ercc8, Ddb1, Cul4a, Usp7, and Lig1 were markedly downregulated in the “HTL (1 mM) + Men1-NC” group. Nevertheless, in the case of concurrent overexpression of Men1 in cells treated with 0 mM or 1 mM HTL, as compared to their corresponding NC groups, there was a noticeable increase in varying degrees for the binding levels of these genes with H3K4me3 (Fig. 3C). These results suggest that the downregulated Menin-H3K4me3 cascade in a high HTL context disrupted the DDR by epigenetically regulating the expressions of DDR pathway-related genes. Thus, overexpressing Menin might alleviate high HTL-induced cellular damage.
Decreased of Menin-H3K4me3 regulates inhibition of DNA damage response pathway in high HTL. A–B Decreased γH2A.X and activation of P-Atr-Chk1 in Men1-overexpressed cells upon 1 mM HTL treatment (n = 3). “NC” denotes that the cell was transfected with an empty plasmid; “ + ” denotes that the cell was transfected with a plasmid overexpressing the Men1 gene. The P values were calculated with one-way ANOVA plus post-hoc test. C ChIP-qPCR analysis of histone H3K4me3 enrichment in different regions of NER pathway genes: Xpa, Ercc8, Ddb1, Cul4a, Usp7, and Lig1. Blank plasmid or Men1 gene overexpression plasmid was transfected into NE4C cells treated with 0 mM HTL and 1 mM HTL, respectively (n = 3). The P values were calculated with one-way ANOVA plus post-hoc test
FA Upregulates the Menin-H3K4me3-DDR Cascade Response
Given that supplementary FA can prevent NTDs [1, 2], we investigated whether FA could alleviate DDR damage induced by HTL, which is distinct from the rescue effect achieved through overexpressing the gene Men1. The results of WB and IF assays demonstrated that when cells undergoing 1 mM HTL treatment were concurrently supplemented with 20 mg/L FA, the adverse effects of HTL (including increased levels of the DNA damage indicator γH2AX, decreased Menin, ph-ATR, and ph-CHK1 protein expression levels, and H3K4me3 modification levels) were significantly reversed. It should also be pointed out that the protein expression levels of Menin, P-Atr, and P-Chk1 have essentially returned to levels comparable to those of the control group (HTL0 + FA0) (Fig. 4A–B; Supplementary Fig. 2B). Moreover, FA also elevated the expressions of genes in the NER pathway (Fig. 4C). These findings collectively suggest that the Menin-H3K4me3-Atr-Chk1-NER pathway might be a therapeutic target for FA supplementation to mitigate the adverse outcomes of high HTL.
Folic acid (FA) activates ph-ATR-Chk1-NER pathway through increasing expression of Menin. A–B WB analysis revealed that supplemented with 10/20 mg/L FA in 1 mM HTL-treated NE4C cells can effectively attenuate DNA damage, elevating Menin protein expression and H3K4me3 modification level and enhancing P-Atr-Chk1 activation (n = 3). The P values were calculated with one-way ANOVA plus post-hoc test. C The down-regulation of NER pathway genes RNA expression caused by high HTL can be counteracted by supplementing with 20 mg/L FA (n = 3). The P values were calculated with unpaired t test
Elevated H3K79hcy Might Regulate the Decreased Expression of Menin in High HTL
Considering the essential role of Menin in the high HTL-induced impairment of the DDR pathway, we investigated how high HTL leads to the downregulation of Menin. High HTL elevated the levels of H3K79hcy modifications in human and chicken embryo models of NTD and epigenetically regulated changes in the expressions of several critical NTD genes [23]. Moreover, Menin is a crucial NTD gene [34], suggesting it might also be regulated by H3K79hcy under high HTL conditions. We retrospectively analyzed published H3K79hcy-ChIP-seq data and found that the three previously validated H3K4me3 modifying transferase genes: Men1 (encoding Menin protein), Wdr82, and Kmt2a (encoding Mll1 protein) were enriched by H3K79hcy (Supplementary Table 6). Through ChIP-qPCR analysis, we confirmed that the binding level of H3K79hcy to Men1 is significantly reduced in cells treated with HTL compared to control cells, while the binding level with Kmt2a does not show a significant change. However, its binding level with Wdr82 has slightly increased (Fig. 5A). This suggested that elevated H3K79hcy might suppress Men1 expression through an epigenetic modification mechanism.
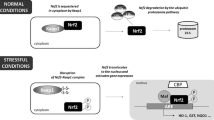
Elevation of H3K79hcy may cause inhibition of Menin in high HTL. A The binding levels of H3K79hcy with Men1, Kmt2a, and Wdr82 genes in different regions were analyzed using ChIP-qPCR (n = 3). The P values were calculated with unpaired t test. B–C Overexpressed Mars in normal cultured NE4C induced elevation of H3K79hcy and reduction of Menin and H3K4me3 (n = 3). The symbol “-” indicates that the cell was not transfected with any plasmid; “NC” denotes that the cell was transfected with an empty plasmid; “ + ” denotes that the cell was transfected with a plasmid overexpressing the Mars gene. The P values were calculated with one-way ANOVA plus post-hoc test. D ChIP-qPCR assays validated reduced enrichment of Men1 with H3K79hcy in Mars overexpressed cells (n = 3). The P values were calculated with unpaired t test. E Pathogenic mechanism diagram of HHcy or high HTL leading to NTDs: The formation of HHcy or high HTL could be due to deficiency of folate. This condition increases the level of H3K79hcy modifications, thus epigenetically regulating the expression of the Men1 gene, leading to a reduction in Menin protein expression. With the downregulation of Menin, the modification level of H3K4me3 also decreases correspondingly. Such changes further suppress the DDR pathway of P-ATR-CHK1-NER and may potentially participate in the molecular mechanism of NTDs. CBS, Cystathionine Beta-Synthase; H3, Histone 3; P, phosphorylation
Next, we overexpressed the Mars gene, which encodes a key enzyme responsible for converting the Hcy to HTL (Fig. 5E) [35], in normally cultured NE4C cells. We observed increased H3K79hcy modification levels and decreased Menin protein expression, accompanied by a reduction in H3K4me3 modification levels (Fig. 5B–C). We used ChIP-qPCR to confirm the enrichment of H3K79hcy to Men1 and Wdr82 genes was significantly reduced compared with the control group after Mars was overexpressed (Fig. 5D). Additionally, we confirmed that overexpression of Mars led to the accumulation of DNA damage (Supplementary Fig. 2). In summary, our results suggest that H3K79hcy epigenetic modifications may be a regulatory mechanism for the decreased expression of Menin in high HTL (Fig. 5E).
Menin-H3K4me3 Levels Are Decreased in HHcy-Related Human Fetal NTD Samples
We selected 10 fetal NTD samples from patients with HHcy (0.0446 ± 0.0038 nmol/mg tissue) in fetal brain tissue and 10 controls with normal Hcy content (0.0036 ± 0.00036) (P < 0.001) (Fig. 6A). WB assay demonstrated that in the HHcy-NTD group, there is a significant decrease in the expression level of Menin protein. Although the expression of phosphorylated ATR exhibited a downward trend, the difference was not statistically significant. However, the phosphorylation of CHK1 has notably increased. Additionally, γH2AX expression levels were significantly elevated, but H3K4me3 was markedly downregulated (Fig. 6B–D). Among the six NER genes, mRNA levels of XPA and ERCC8 were decreased; however, levels of DDB1 and LIG1 were increased, and no significant changes were observed for CUL4A and USP7 (Fig. 6E). These results partially describe the mechanism of ATR inactivation in HHcy-induced NTDs.
Validation of decreased protein expression of MENIN and P-ATR in HHcy-related human fetal NTDs. A Hcy levels in 10 normals and 10 NTDs from human fetal brain tissues. B–C Decreased of MENIN, whereas increased P-CHK1 protein expression and the decreasing trend of P-ATR in HHcy-related human fetal NTDs (n = 10). D WB validated an increased of γH2A.X and decreased of H3K4me3 in HHcy related human fetal NTDs (n = 5). E RT-qPCR assays for detection mRNA level of NER pathway genes: XPA, ERCC8, DDB1, CUL4A, USP7, and LIG1 in brain tissues of human fetal NTDs (n = 5). All the P values were calculated with unpaired t test
Discussion
Elevated maternal homocysteine levels (hyperhomocysteinemia, HHcy) contribute to an increased incidence of fetal NTDs [1]. Our study demonstrated that the expression of Menin was markedly downregulated in high HTL-induced chicken embryos and HHcy-related human NTD brain tissue samples. This leads to the further disruption of the ATR-Chk1 signaling pathway and Xpa-mediated NER, a crucial DDR mechanism, via H3K4me3-mediated epigenetic regulation. Moreover, Menin itself may be subjected to epigenetic modulation by H3K79hcy (Fig. 5E). Collectively, these findings shed light on the underlying molecular mechanisms through which HHcy/high HTL promotes the development of NTDs.
In this study, we initially observed an escalation in DNA damage in E4 and E5 chicken embryos, induced by high HTL treatment; moreover, the expression levels of DNA damage repair pathway genes such as Atr, Chek1, Xpa, and Ercc8 were significantly reduced in chicken embryos at the E5 stage; subsequently, γH2AX was augmented in NE4C cells with high HTL, and the DDR pathway (Atr-Chk1-NER) was compromised. Additionally, we discovered a decline in P-ATR and downregulation of XPA and ERCC8 gene expressions in human fetal NTDs associated with HHcy. Collectively, high HTL/HHcy resulted in an increase in DNA damage in vitro and in vivo, as well as disruption of the P-ATR-related NER pathway (Fig. 1; Supplementary Figs. 1 and 6). However, in human fetal NTDs associated with HHcy, the expression of P-CHK1 protein was enhanced and DDB1 and LIG1 mRNA were upregulated, results that are contrary to those obtained for in vitro NE4C with high HTL; Moreover, this difference can also be seen in the E5 stage chicken embryos induced by high HTL, reflected in that the mRNA expression levels of pathway genes Ddb1, Usp7, and Lig1 do not change (Supplementary Fig. 1B). We hypothesize that the observed diversity in the expression of DDR pathway genes under high HTL/HHcy conditions across various species is due to two reasons. First, although high HTL is a significant route for intrabody HHcy, other paths exist for HHcy, such as its conversion into cysteine by cystathionine β-synthase or its regeneration into methionine via methionine synthase (Fig. 5E). This suggests that a high HTL environment cannot fully simulate a HHcy environment. Second, the inherent heterogeneity and differences in developmental stages within organisms—for instance, inconsistent time points for gene testing—might contribute to the variations in experimental results. In this study, conducting such tests in vitro in mouse NE4C at the E9 stage (i.e., when the mouse is in the neural tube closure period [36]) is vastly different from tests conducted in chicken embryos at the post-incubation E5 stage (the neural tube closure period for chicken embryos is begin within 33 to 36 h after incubation and complete within 48 to 72 h [37]). Not to mention, the NTD fetal samples we collected at approximately 20 weeks of gestation, which surpasses the stage of neural tube closure (usually commencing 25–27 days after fertilization and completed between 28–30 days [36]). Therefore, the difference in DDR gene expression may be due to chicken embryos and human samples being at significantly mature developmental stages. Despite these differences, the increase in DNA damage caused by high HTL/HHcy and the dysregulation of the DDR pathway are widely observed pathological phenomena both in vivo and in vitro.
Subsequently, we found that Menin expression was inhibited under high HTL/HHcy conditions and H3K4me3 modification levels were diminished, exerting a pivotal regulatory function related to the disturbance of the Atr-Chk1-NER pathway (Figs. 2, 3 and 6). Moreover, we attempted to mitigate the adverse impacts of high HTL by gene therapy and nutritional supplementation approaches; the results demonstrated that Menin overexpression or FA supplementation enhanced DDR via H3K4me3 epigenetic regulatory mechanisms (Figs. 3 and 4). Finally, we investigated the mechanisms related to the reduction of Menin under high HTL conditions and postulated that H3K79hcy [23] might have a negative effect on the epigenetic regulation of Men1/Menin expression (Fig. 5). Nevertheless, further exploration is required to ascertain whether other mechanisms modulate the aberrant expression of Men1/Menin and if additional histone modification mechanisms participate in the disruption of DNA damage repair responses in high HTL/HHcy.
The protein Menin, encoded by the Men1 gene, is predominantly a nuclear scaffold protein that has a crucial role in embryonic development. When the Men1 gene is a null mutant in mice, a significant proportion of embryos exhibit neural tube exposure, leading to NTDs [34]. The pathological mechanism of menin-induced NTDs remains unclear, but currently, our understanding of the functional mechanisms of Menin can be summarized as follows: (1) Menin interacts with various proteins to regulate gene transcription levels: it associates with transcription activators such as c-myb and H3K4me3 methyltransferases such as Mll2; with transcription repressors such as deacetylase Sirt1 and H3K27me3 modifying enzyme EZH2; and with key molecules of critical signaling pathways involved in neural tube closure, such as β-catenin in the Wnt pathway [38] and PRMT5 protein in the SHH pathway [39], thereby regulating gene expression or pathway activity [40, 41]. (2) Menin participates in DNA damage repair: in pancreatic neuroendocrine tumors or lung cancer tissues, the low expression of Menin triggered abnormal DNA damage responses, increased γH2AX staining, inhibited the p-ATR pathway, and activated the p-ATM pathway to maintain genomic stability [32]. In our study, we found that in high HTL-induced NTD, low Menin expression further suppressed H3K4me3, which regulated the expressions of genes associated with the Atr-Chk1-NER DNA damage repair pathways. This research further enhances our understanding of the Menin-regulated DDR mechanism and gene expression. However, in high HTL/HHcy-induced NTDs, the expression pattern and regulatory mechanisms of Menin are still unclear.
We previously identified elevated H3K79hcy modification levels in high HTL-induced NTD chicken embryos, which led to the inhibition of NTD-associated gene expression [23]. Building on this work, through the in vitro overexpression of the Mars gene to upregulate H3K79hcy modification, we confirmed its crucial epigenetic regulatory role in Menin expression. It is important to consider that an excessive accumulation of AdoHcy (a precursor of Hcy within one-carbon metabolism) can be detrimental because it inhibits most methyltransferases. This indicates that HHcy might also repress Menin expression via this mechanism [42]. Moreover, although Hcy is a non-protein amino acid, HHcy-HTL triggers protein homocysteinylation, a post-translational modification that results in proteins losing their inherent biological function leading to adverse effects on various disease phenotypes [43]. Many novel N-homocysteinylated protein modifications have been reported in NTDs, including N-homocysteinylated SOD1/2 [13]. Although there is no direct evidence for Menin homocysteinylation, Menin can undergo SUMOylation, phosphorylation, and ubiquitylation [40]. Consequently, we propose that the N-homocysteinylation of Menin might represent an alternative regulatory mechanism contributing to the reduction of Menin expression in high HTL-induced NTDs and, thus, warrants further study.
There is interplay between H3K79hcy and H3K4me3 and interactions between histone post-translational modifications have been reported for H3K4me3 and H3K27ac [44], as well as H3K4me3 and H4K16ac [45], indicating crosstalk between epigenetic mechanisms is common. In contrast to H3K4me3, which is primarily located at active promoters, enhancers, or near transcription start sites and is involved in gene activation, H3K79hcy preferably resides within gene bodies and is mainly associated with gene repression [23, 46]. Our findings demonstrated that there is a concomitant upregulation of H3K79hcy and downregulation of H3K4me in HHcy-induced NTDs, suggesting a potential interplay between these two modifications. Furthermore, high HTL treatment or Mars overexpression elevated H3K79hcy levels but downregulated Men1 expression via negative epigenetic regulation. This, in turn, led to a decrease in Menin protein expression and subsequent reduction in H3K4me3 modification. Considering that Menin itself can bind to histone-modifying enzymes and affect other histone modification levels [40, 47], this raises the question of whether menin might reciprocally influence H3K79hcy levels. This possibility warrants further investigation. However, the underlying mechanism by which this crosstalk is facilitated through menin remains to be elucidated.
There are some limitations in this study that need to be noted. Firstly, in this study, we investigated spina bifida malformations in chicken embryos and human embryos. Due to the relative ease of acquiring brain tissue over spinal cord tissue, their brain tissues were used as research subjects. However, some literature suggests that despite both brain and spinal cord tissues being parts of the central nervous system, their developmental mechanisms may be independent and differ from one another [48]. Furthermore, different NTD phenotypes may arise from distinct etiologies [49]. In light of this, more rigorous approaches should be adopted when selecting and validating models in the future. Secondly, the number of human fetal NTD samples related to HHcy that we have collected is relatively small, so it is necessary to further increase the sample size to consolidate our research findings. Thirdly, whether the dysregulated expression of P-ATR-CHK1 under high HTL or HHcy conditions is also subject to regulation by the Menin-H3K4ME3 mechanism or there exist other regulatory mechanisms (Fig. 5E) is worth further exploration.
In summary, the present study reports that the downregulation of Menin during embryonic development epigenetically modulated the P-Atr-Chk1-NER DNA damage repair pathway through H3K4me3, providing novel insights into the pathogenesis of HHcy-induced NTDs. The investigation of Menin protein offers potential therapeutic targets for the prevention and treatment of NTDs. Furthermore, the regulatory role of H3K79hcy on H3K4me3 provides new evidence of crosstalk between histone epigenetic modification mechanisms.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Smith AD, Refsum H (2021) Homocysteine - from disease biomarker to disease prevention. J Intern Med 290(4):826–854
D’Souza SW, Glazier JD (2022) Homocysteine metabolism in pregnancy and developmental impacts. Front Cell Dev Biol 10:802285
Jakubowski H, Głowacki R (2011) Chemical biology of homocysteine thiolactone and related metabolites. Adv Clin Chem 55:81–103
Li RL, Zhao WW, Gao BY (2018) Advanced glycation end products induce neural tube defects through elevating oxidative stress in mice. Neural Regen Res 13(8):1368–1374
Wang X, Yue H, Li S, Guo J, Guan Z, Zhu Z et al (2021) Genetic polymorphisms in DNA repair gene APE1/Ref-1 and the risk of neural tube defects in a high-risk area of China. Reprod Sci 28(9):2592–2601
Albino D, Brizzolara A, Moretti S, Falugi C, Mirisola V, Scaruffi P et al (2011) Gene expression profiling identifies eleven DNA repair genes down-regulated during mouse neural crest cell migration. Int J Dev Biol 55(1):65–72
Lee MS, Bonner JR, Bernard DJ, Sanchez EL, Sause ET, Prentice RR et al (2012) Disruption of the folate pathway in zebrafish causes developmental defects. BMC Dev Biol 12:12
Hayden MR, Tyagi SC (2004) Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J 3:4
Koz ST, Gouwy NT, Demir N, Nedzvetsky VS, Etem E, Baydas G (2010) Effects of maternal hyperhomocysteinemia induced by methionine intake on oxidative stress and apoptosis in pup rat brain. Int J Dev Neurosci 28(4):325–329
Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L et al (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20(18):6920–6926
Zhang HS, Cao EH, Qin JF (2005) Homocysteine induces cell cycle G1 arrest in endothelial cells through the PI3K/Akt/FOXO signaling pathway. Pharmacology 74(2):57–64
Wang R, Han ZJ, Song G, Cui Y, Xia HF, Ma X (2021) Homocysteine-induced neural tube defects in chick embryos via oxidative stress and DNA methylation associated transcriptional down-regulation of miR-124. Toxicol Res 10(3):425–435
Mei X, Qi D, Zhang T, Zhao Y, Jin L, Hou J et al (2020) Inhibiting MARSs reduces hyperhomocysteinemia-associated neural tube and congenital heart defects. EMBO Mol Med 12(3):e9469
Wang D, Zhao R, Qu YY, Mei XY, Zhang X, Zhou Q et al (2018) Colonic lysine homocysteinylation induced by high-fat diet suppresses DNA damage repair. Cell Rep 25(2):398-412.e6
Li J, Zhao H, McMahon A, Yan S (2022) APE1 assembles biomolecular condensates to promote the ATR-Chk1 DNA damage response in nucleolus. Nucleic Acids Res 50(18):10503–10525
Saldivar JC, Cortez D, Cimprich KA (2017) The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat Rev Mol Cell Biol 18(10):622–636
Kang TH (2021) Circadian rhythm of NER and ATR pathways. Biomolecules 11(5):715
Jarrett SG, Wolf Horrell EM, D’Orazio JA (2016) AKAP12 mediates PKA-induced phosphorylation of ATR to enhance nucleotide excision repair. Nucleic Acids Res 44(22):10711–10726
Shuck SC, Short EA, Turchi JJ (2008) Eukaryotic nucleotide excision repair: from understanding mechanisms to influencing biology. Cell Res 18(1):64–72
Fousteri M, Mullenders LH (2008) Transcription-coupled nucleotide excision repair in mammalian cells: molecular mechanisms and biological effects. Cell Res 18(1):73–84
Jakubowski H (2019) Protein N-homocysteinylation and colorectal cancer. Trends Cancer 5(1):7–10
Fryer AA, Emes RD, Ismail KM, Haworth KE, Mein C, Carroll WD et al (2011) Quantitative, high-resolution epigenetic profiling of CpG loci identifies associations with cord blood plasma homocysteine and birth weight in humans. Epigenetics 6(1):86–94
Zhang Q, Bai B, Mei X, Wan C, Cao H, Dan L et al (2018) Elevated H3K79 homocysteinylation causes abnormal gene expression during neural development and subsequent neural tube defects. Nat Commun 9(1):3436
Perła-Kaján J, Jakubowski H (2019) Dysregulation of epigenetic mechanisms of gene expression in the pathologies of hyperhomocysteinemia. Int J Mol Sci 20(13):3140
Taparia S, Gelineau-van Waes J, Rosenquist TH, Finnell RH (2007) Importance of folate-homocysteine homeostasis during early embryonic development. Clin Chem Lab Med 45(12):1717–1727
Denny KJ, Kelly CF, Kumar V, Witham KL, Cabrera RM, Finnell RH et al (2016) Autoantibodies against homocysteinylated protein in a mouse model of folate deficiency-induced neural tube defects. Birth Defects Res A 106(3):201–207
Li Dan, Wan Chunlei, Bai Baoling, Cao Haiyan, Liu Changyun, Zhang Q (2018) HTL induced neural tube defects in chick embryos. Chinese Journal of Birth Health and Heredity 26(5):89–91
Zhang Qin, Li Dan, Bai Baoling, Wan Chunlei, Xiao Z (2020) ITRAQ proteomics revealed that abnormal expressed protein of oxidative phosphorylation pathway was involved in the development of neural tube defects in chicken embryos. Chinese Journal of Reproductive Health 31(3):227–32
Xie Q, Li C, Song X, Wu L, Jiang Q, Qiu Z et al (2017) Folate deficiency facilitates recruitment of upstream binding factor to hot spots of DNA double-strand breaks of rRNA genes and promotes its transcription. Nucleic Acids Res 45(5):2472–2489
Shechter D, Dormann HL, Allis CD, Hake SB (2007) Extraction, purification and analysis of histones. Nat Protoc 2(6):1445–1457
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL et al (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26(3):317–325
Qiu H, Jin BM, Wang ZF, Xu B, Zheng QF, Zhang L et al (2020) MEN1 deficiency leads to neuroendocrine differentiation of lung cancer and disrupts the DNA damage response. Nat Commun 11(1):1009
Zhang C, Guan Y, Zou J, Yang X, Bayliss G, Zhuang S (2022) Histone methyltransferase MLL1 drives renal tubular cell apoptosis by p53-dependent repression of E-cadherin during cisplatin-induced acute kidney injury. Cell Death Dis 13(9):770
Bertolino P, Radovanovic I, Casse H, Aguzzi A, Wang ZQ, Zhang CX (2003) Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech Dev 120(5):549–560
Senger B, Despons L, Walter P, Jakubowski H, Fasiolo F (2001) Yeast cytoplasmic and mitochondrial methionyl-tRNA synthetases: two structural frameworks for identical functions. J Mol Biol 311(1):205–216
Greene ND, Copp AJ (2014) Neural tube defects. Annu Rev Neurosci 37:221–242
Williams RM, Lukoseviciute M, Sauka-Spengler T, Bronner ME (2022) Single-cell atlas of early chick development reveals gradual segregation of neural crest lineage from the neural plate border during neurulation. eLife 11:e74464
Shi DL (2022) Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure. Cell Mol Life Sci 79(12):586
Brooks ER, Islam MT, Anderson KV, Zallen JA (2020) Sonic hedgehog signaling directs patterned cell remodeling during cranial neural tube closure. eLife 9:e60234
Matkar S, Thiel A, Hua X (2013) Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci 38(8):394–402
Ehrlich L, Hall C, Meng F, Lairmore T, Alpini G, Glaser S (2017) A review of the scaffold protein menin and its role in hepatobiliary pathology. Gene Expr 17(3):251–263
Kim J, Kim H, Roh H, Kwon Y (2018) Causes of hyperhomocysteinemia and its pathological significance. Arch Pharmacal Res 41(4):372–383
Chen SM, Tang XQ (2021) Homocysteinylation and sulfhydration in diseases. Curr Neuropharmacol 20(9):1726–1735
Zhao W, Xu Y, Wang Y, Gao D, King J, Xu Y et al (2021) Investigating crosstalk between H3K27 acetylation and H3K4 trimethylation in CRISPR/dCas-based epigenome editing and gene activation. Sci Rep 11(1):15912
Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W et al (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138(5):1019–1031
Soares LM, He PC, Chun Y, Suh H, Kim T, Buratowski S (2017) Determinants of histone H3K4 methylation patterns. Mol Cell 68(4):773–85.e6
Kim H, Lee JE, Cho EJ, Liu JO, Youn HD (2003) Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res 63(19):6135–6139
Henrique D, Abranches E, Verrier L, Storey KG (2015) Neuromesodermal progenitors and the making of the spinal cord. Development 142(17):2864–2875
Harris MJ, Juriloff DM (2010) An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A 88(8):653–669
Acknowledgements
We thank J. Ludovic Croxford, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the language of a draft of this manuscript.
Funding
The work was supported by the National Natural Science Foundations of China (81901167) to Baoling Bai and also by a grant from the National Natural Science Foundation of China (81971397 and 82171652), the Beijing Natural Science Foundation (7232008, 7222017), the Public Service Development and Reform Pilot Project of Beijing Medical Research Institute (BMR2019-11), and the Research Foundation of the Capital Institute of Pediatrics (CXYJ-2021–02) to Qin Zhang. In addition, Ting Zhang was supported by the Nature Science Fund (81971390) and CAMS Initiative for Innovative Medicine (CAMS-I2M -1–008).
Author information
Authors and Affiliations
Contributions
Q.Z. and B.B. obtained funding. Q.Z. and B.B. conceived the study and designed the experiments. B.B., C.W., Z.X., D.L., L.L., and K.Z. conducted the experiments. B.B. and K.Z. analyzed the data. C.W., D.L., and L.L. interpreted the data. B.B. and Q.Z. wrote the manuscript. T.Z. and Q.Z. edited the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The human subjects involved in this study obtained informed consent; the study was approved by the Capital Institute of Pediatrics (Beijing, China) (SHERLLM2014002) and was carried out according to the Declaration of Helsinki protocols. Animal research was approved by the animal ethics committee of the Capital Institute of Pediatrics (DWLL2020001).
Consent to Participate
All subjects were given informed consent.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Zhang and Qin Zhang are co-correspondents.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, B., Wan, C., Xiao, Z. et al. High Homocysteine-Thiolactone Leads to Reduced MENIN Protein Expression and an Impaired DNA Damage Response: Implications for Neural Tube Defects. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04033-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04033-7