Abstract
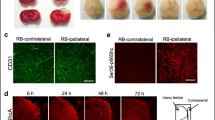
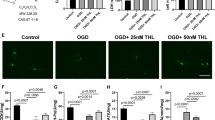
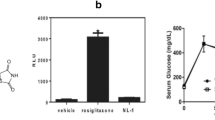
Ischemic stroke is one of the major causes of morbidity and mortality worldwide. Mitochondria play a vital role in the pathological processes of cerebral ischemic injury, but its transplantation and underlying mechanisms remain unclear. In the present study, we examined the effects of mitochondrial therapy on the modulation of AMPK and SIRT1/PGC-1α signaling pathway, oxidative stress, and NLRP3 inflammasome activation after photothrombotic ischemic stroke (pt-MCAO). The adult male mice were subjected to the pt-MCAO in which the proximal-middle cerebral artery was exposed with a 532-nm laser beam for 4 min by retro-orbital injection of a photosensitive dye (Rose Bengal: 15 mg/kg) before the laser light exposure and isolated mitochondria (100 μg protein) were administered intranasally at 30 min, 24 h, and 48 h following post-stroke. After 72 h, mice were tested for neurobehavioral outcomes and euthanized for infarct volume, brain edema, and molecular analysis. First, we found that mitochondria therapy significantly decreased brain infarct volume and brain edema, improved neurological dysfunction, attenuated ischemic stroke-induced oxidative stress, and neuroinflammation. Second, mitochondria treatment inhibited NLRP3 inflammasome activation. Finally, mitochondria therapy accelerated p-AMPKα(Thr172) and PGC-1α expression and resorted SIRT1 protein expression levels in pt-MCAO mice. In conclusion, our results demonstrate that mitochondria therapy exerts neuroprotective effects by inhibiting oxidative damage and inflammation, mainly dependent on the heightening activation of the AMPK and SIRT1/PGC-1α signaling pathway. Thus, intranasal delivery of mitochondria might be considered a new therapeutic strategy for ischemic stroke treatment.










Similar content being viewed by others
Data Availability
The data generated and analyzed in this study are available from the corresponding author upon reasonable request.
References
Feigin VL, Stark BA, Johnson CO et al (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20:795–820. https://doi.org/10.1016/S1474-4422(21)00252-0
Kaushik P, Ali M, Salman M et al (2021) Harnessing the mitochondrial integrity for neuroprotection: therapeutic role of piperine against experimental ischemic stroke. Neurochem Int 149:105138. https://doi.org/10.1016/j.neuint.2021.105138
Cheng NT, Kim AS (2015) Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist 5:101–109. https://doi.org/10.1177/1941874415583116
Papanagiotou P, Ntaios G (2018) Endovascular thrombectomy in acute ischemic stroke. Circ Cardiovasc Interv 11. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005362
Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y (2018) Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol 16:1396–1415. https://doi.org/10.2174/1570159X16666180302115544
Qin C, Yang S, Chu Y-H et al (2022) Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 7:215. https://doi.org/10.1038/s41392-022-01064-1
Russo E, Nguyen H, Lippert T et al (2018) Mitochondrial targeting as a novel therapy for stroke. Brain Circ 4:84. https://doi.org/10.4103/bc.bc_14_18
He Z, Ning N, Zhou Q et al (2020) Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med 146:45–58. https://doi.org/10.1016/j.freeradbiomed.2019.11.005
Carinci M, Vezzani B, Patergnani S et al (2021) Different roles of mitochondria in cell death and inflammation: focusing on mitochondrial quality control in ischemic stroke and reperfusion. Biomedicines 9:169. https://doi.org/10.3390/biomedicines9020169
Niizuma K, Endo H, Chan PH (2009) Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem 109:133–138. https://doi.org/10.1111/j.1471-4159.2009.05897.x
Zádori D, Klivényi P, Szalárdy L et al (2012) Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J Neurol Sci 322:187–191. https://doi.org/10.1016/j.jns.2012.06.004
Chen S-D, Yang D-I, Lin T-K et al (2011) Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215. https://doi.org/10.3390/ijms12107199
Song M, Zhou Y, Fan X (2022) Mitochondrial quality and quantity control: mitophagy is a potential therapeutic target for ischemic stroke. Mol Neurobiol 59:3110–3123. https://doi.org/10.1007/s12035-022-02795-6
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022
Zhao Y, Liu X, Zheng Y et al (2021) Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT-1/NF-κB signaling pathway and gut microbiota. Sci Rep 11:20558. https://doi.org/10.1038/s41598-021-00071-6
Gravandi MM, Fakhri S, Zarneshan SN et al (2021) Flavonoids modulate AMPK/PGC-1α and interconnected pathways toward potential neuroprotective activities. Metab Brain Dis 36:1501–1521. https://doi.org/10.1007/s11011-021-00750-3
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L et al (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15(5):675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Cantó C, Gerhart-Hines Z, Feige JN et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT-1 activity. Nature 458:1056–1060. https://doi.org/10.1038/nature07813
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90. https://doi.org/10.1210/er.2002-0012
Duncan JG (2011) Peroxisome proliferator activated receptor-alpha (PPARα) and PPAR gamma coactivator-1alpha (PGC-1α) regulation of cardiac metabolism in diabetes. Pediatr Cardiol 32:323–328. https://doi.org/10.1007/s00246-011-9889-8
Selvakumar GP, Iyer SS, Kempuraj D et al (2018) Glia maturation factor dependent inhibition of mitochondrial PGC-1α triggers oxidative stress-mediated apoptosis in N27 rat dopaminergic neuronal cells. Mol Neurobiol 55:7132–7152. https://doi.org/10.1007/s12035-018-0882-6
Falany CN (1997) Enzymology of human cytosolic sulfotransferases. FASEB J 11:206–216. https://doi.org/10.1096/fasebj.11.4.9068609
Allali-Hassani A, Pan PW, Dombrovski L et al (2007) Structural and chemical profiling of the human cytosolic sulfotransferases. PLoS Biol 5:e97. https://doi.org/10.1371/journal.pbio.0050097
Brettrager EJ, Meehan AW, Falany CN, van Waardenburg RCAM (2022) Sulfotransferase 4A1 activity facilitates sulfate-dependent cellular protection to oxidative stress. Sci Rep 12:1625. https://doi.org/10.1038/s41598-022-05582-4
Brennan MD, Condra J (2005) Transmission disequilibrium suggests a role for the sulfotransferase-4A1 gene in schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet 139B:69–72. https://doi.org/10.1002/ajmg.b.30222
Phelan K, McDermid HE (2011) The 22q13.3 deletion syndrome (Phelan-McDermid syndrome). Mol Syndromol 2:186–201. https://doi.org/10.1159/000334260
Garcia PL, Hossain MI, Andrabi SA, Falany CN (2018) Generation and characterization of SULT4A1 mutant mouse models. Drug Metab Dispos 46:41–45. https://doi.org/10.1124/dmd.117.077560
Hossain MI, Marcus JM, Lee JH et al (2019) SULT4A1 protects against oxidative-stress induced mitochondrial dysfunction in neuronal cells. Drug Metab Dispos 47:949–953. https://doi.org/10.1124/dmd.119.088047
Hayakawa K, Esposito E, Wang X et al (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535:551–555. https://doi.org/10.1038/nature18928
Nakamura Y, Lo EH, Hayakawa K (2020) Placental mitochondria therapy for cerebral ischemia-reperfusion injury in mice. Stroke 51:3142–3146. https://doi.org/10.1161/STROKEAHA.120.030152
Huang P-J, Kuo C-C, Lee H-C et al (2016) Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transplant 25:913–927. https://doi.org/10.3727/096368915X689785
Zhang Z, Ma Z, Yan C et al (2019) Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res 356:322–331. https://doi.org/10.1016/j.bbr.2018.09.005
Pourmohammadi-Bejarpasi Z, Roushandeh AM, Saberi A et al (2020) Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res Bull 165:70–80. https://doi.org/10.1016/j.brainresbull.2020.09.018
Ahmed HA, Ismael S, Salman M et al (2022) Direct AT2R stimulation slows post-stroke cognitive decline in the 5XFAD Alzheimer’s disease mice. Mol Neurobiol 59:4124–4140. https://doi.org/10.1007/s12035-022-02839-x
Preble JM, Pacak CA, Kondo H et al (2014) Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp. https://doi.org/10.3791/51682
Ismael S, Nasoohi S, Li L et al (2021) Thioredoxin interacting protein regulates age-associated neuroinflammation. Neurobiol Dis 156:105399. https://doi.org/10.1016/j.nbd.2021.105399
Nasoohi S, Tayefeh Ghahremani P, Alehossein P et al (2023) The p75 neurotrophin receptor inhibitor, LM11A-31, ameliorates acute stroke injury and modulates astrocytic proNGF. Exp Neurol 359:114161. https://doi.org/10.1016/j.expneurol.2022.114161
Salman M, Tabassum H, Parvez S (2020) Tannic acid provides neuroprotective effects against traumatic brain injury through the PGC-1α/Nrf2/HO-1 pathway. Mol Neurobiol 57:2870–2885. https://doi.org/10.1007/s12035-020-01924-3
Salman M, Ismael S, Li L et al (2021) Endothelial thioredoxin-interacting protein depletion reduces hemorrhagic transformation in hyperglycemic mice after embolic stroke and thrombolytic therapy. Pharmaceuticals 14:983. https://doi.org/10.3390/ph14100983
Ismael S, Patrick D, Salman M et al (2022) Verapamil inhibits TXNIP-NLRP3 inflammasome activation and preserves functional recovery after intracerebral hemorrhage in mice. Neurochem Int 161:105423. https://doi.org/10.1016/j.neuint.2022.105423
Xiao X-T, Luo C, Yuan Y et al (2021) Systematic evaluation during early-phase ischemia predicts outcomes in middle cerebral artery occlusion mice. Neuroreport 32:29–37. https://doi.org/10.1097/WNR.0000000000001553
Ruderman NB, Julia XX, Nelson L et al (2010) AMPK and SIRT-1: a long-standing partnership? Am J Physiol Metab 298:E751–E760. https://doi.org/10.1152/ajpendo.00745.2009
Zhou Y, Wang S, Li Y et al (2018) SIRT-1/PGC-1α signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00443
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. https://doi.org/10.1042/bse0470069
Hong P, Gu R-N, Li F-X et al (2019) NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J Neuroinflammation 16:121. https://doi.org/10.1186/s12974-019-1498-0
Salman M, Tabassum H, Parvez S (2020) Nrf2/HO-1 mediates neuroprotective effects of pramipexole by attenuating oxidative damage and mitochondrial perturbation after traumatic brain injury. Dis Model Mech. https://doi.org/10.1242/dmm.045021
Li J, Gao W, Zhao Z et al (2022) Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol Neurobiol 59:2855–2873. https://doi.org/10.1007/s12035-022-02740-7
Luo Y, Fang Y, Kang R et al (2020) Inhibition of EZH2 (enhancer of zeste homolog 2) attenuates neuroinflammation via H3k27me3/SOCS3/TRAF6/NF-κB (trimethylation of histone 3 lysine 27/suppressor of cytokine signaling 3/tumor necrosis factor receptor family 6/nuclear factor-κB) in a rat mode. Stroke 51:3320–3331. https://doi.org/10.1161/STROKEAHA.120.029951
Cherait A, Maucotel J, Lefranc B et al (2021) Intranasal administration of PACAP is an efficient delivery route to reduce infarct volume and promote functional recovery after transient and permanent middle cerebral artery occlusion. Front Endocrinol (Lausanne) 11. https://doi.org/10.3389/fendo.2020.585082
Guo X, Zhang Y, Liu C et al (2022) Intranasal administration of β-1, 3-galactosyltransferase 2 confers neuroprotection against ischemic stroke by likely inhibiting oxidative stress and <scp>NLRP3</scp> inflammasome activation. FASEB J 36. https://doi.org/10.1096/fj.202200456RR
Salman M, Ismael S, Li L et al (2022) Acute hyperglycemia exacerbates hemorrhagic transformation after embolic stroke and reperfusion with tPA: a possible role of TXNIP-NLRP3 inflammasome. J Stroke Cerebrovasc Dis 31:106226. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106226
Ismael S, Nasoohi S, Yoo A et al (2020) Tissue plasminogen activator promotes TXNIP-NLRP3 inflammasome activation after hyperglycemic stroke in mice. Mol Neurobiol 57:2495–2508. https://doi.org/10.1007/s12035-020-01893-7
Ishrat T, Soliman S, Eldahshan W et al (2018) Silencing VEGF-B diminishes the neuroprotective effect of candesartan treatment after experimental focal cerebral ischemia. Neurochem Res 43:1869–1878. https://doi.org/10.1007/s11064-018-2604-x
Lee J-Y, Castelli V, Kumar N et al (2022) Contraceptive drug, Nestorone, enhances stem cell-mediated remodeling of the stroke brain by dampening inflammation and rescuing mitochondria. Free Radic Biol Med 183:138–145. https://doi.org/10.1016/j.freeradbiomed.2022.03.020
Chen H, Yoshioka H, Kim GS et al (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517. https://doi.org/10.1089/ars.2010.3576
Garry PS, Ezra M, Rowland MJ et al (2015) The role of the nitric oxide pathway in brain injury and its treatment — from bench to bedside. Exp Neurol 263:235–243. https://doi.org/10.1016/j.expneurol.2014.10.017
Toda N, Ayajiki K, Okamura T (2009) Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 61:62–97. https://doi.org/10.1124/pr.108.000547
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136. https://doi.org/10.1093/jnen/62.2.127
Ismael S, Zhao L, Nasoohi S, Ishrat T (2018) Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 8:5971. https://doi.org/10.1038/s41598-018-24350-x
Yang-Wei Fann D, Lee S-Y, Manzanero S et al (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4:e790–e790. https://doi.org/10.1038/cddis.2013.326
Yang F, Wang Z, Wei X et al (2014) NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 34:660–667. https://doi.org/10.1038/jcbfm.2013.242
Lin J-N, Lin VC-H, Rau K-M et al (2010) Resveratrol modulates tumor cell proliferation and protein translation via SIRT-1-dependent AMPK activation. J Agric Food Chem 58:1584–1592. https://doi.org/10.1021/jf9035782
Wang P, Xu T-Y, Guan Y-F et al (2011) Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT-1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol 69:360–374. https://doi.org/10.1002/ana.22236
Wareski P, Vaarmann A, Choubey V et al (2009) PGC-1α and PGC-1Β regulate mitochondrial density in neurons. J Biol Chem 284:21379–21385. https://doi.org/10.1074/jbc.M109.018911
Awad-Igbaria Y, Ferreira N, Keadan A, Sakas R, Edelman D, Shamir A, Francous-Soustiel J et al (2023) HBO treatment enhances motor function and modulates pain development after sciatic nerve injury via protection the mitochondrial function. J Transl Med. 15:545. https://doi.org/10.1186/s12967-023-04414-x
Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P (2008) Metabolic adaptations through the PGC-1α and SIRT-1 pathways. FEBS Lett 582:46–53. https://doi.org/10.1016/j.febslet.2007.11.034
Wang H, Peiris TH, Mowery A et al (2008) CCAAT/enhancer binding protein-β is a transcriptional regulator of peroxisome-proliferator-activated receptor-γ coactivator-1α in the regenerating liver. Mol Endocrinol 22:1596–1605. https://doi.org/10.1210/me.2007-0388
Han L, Wang P, Zhao G et al (2013) Upregulation of SIRT-1 by 17β-estradiol depends on ubiquitin-proteasome degradation of PPAR-γ mediated by NEDD4-1. Protein Cell 4:310–321. https://doi.org/10.1007/s13238-013-2124-z
Fu B, Zhang J, Zhang X et al (2014) Alpha-lipoic acid upregulates SIRT-1-dependent PGC-1α expression and protects mouse brain against focal ischemia. Neuroscience 281:251–257. https://doi.org/10.1016/j.neuroscience.2014.09.058
Chandrasekaran K, Anjaneyulu M, Choi J et al (2019) Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD+-dependent SIRT-1–PGC-1α–TFAM pathway. Int Rev Neurobiol 145:177–209. https://doi.org/10.1016/bs.irn.2019.04.002
Gao Z, Zhu Q, Zhang Y et al (2013) Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol Neurobiol 48:690–701. https://doi.org/10.1007/s12035-013-8460-4
Chamorro Á, Dirnagl U, Urra X, Planas AM (2016) Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 15:869–881. https://doi.org/10.1016/S1474-4422(16)00114-9
Wang Q, Tang X, Yenari M (2007) The inflammatory response in stroke. J Neuroimmunol 184:53–68. https://doi.org/10.1016/j.jneuroim.2006.11.014
Chen J, Zhang M, Zhang X et al (2019) EZH2 inhibitor DZNep modulates microglial activation and protects against ischaemic brain injury after experimental stroke. Eur J Pharmacol 857:172452. https://doi.org/10.1016/j.ejphar.2019.172452
He T, Shang J, Gao C et al (2021) A novel SIRT6 activator ameliorates neuroinflammation and ischemic brain injury via EZH2/FOXC1 axis. Acta Pharm Sin B 11:708–726. https://doi.org/10.1016/j.apsb.2020.11.002
Korzhevskii DE, Kirik OV (2016) Brain microglia and microglial markers. Neurosci Behav Physiol 46:284–290. https://doi.org/10.1007/s11055-016-0231-z
Yew WP, Djukic ND, Jayaseelan JSP et al (2019) Early treatment with minocycline following a stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J Neuroinflammation 16:6. https://doi.org/10.1186/s12974-018-1379-y
Code Availability
Not applicable
Funding
This work was supported by the UTHSC Bridge funding award (E073005058– Bridge Support-2022) and the National Institute of Health (R01-NS097800 and R56 NS127924-01) to T.I and DK117183 and DK132230 to A.B. The American Heart Association (AHA) Postdoctoral Fellowship 1029163 to M.S.
Author information
Authors and Affiliations
Contributions
Mohd. Salman conducted all experiments, data analysis, writing of the first draft, and manuscript preparation. Amandeep Bajwa reviewed the manuscript. Amanda Stayton helped in mitochondria isolation and preparation and reviewed the manuscript. Kehkashan Parveen, Arshi Parveen, Michelle A Puchowicz, and Suhel Parvez helped with the experimental design and review of the manuscript. Tauheed Ishrat designed and oversaw the project, including experimental design, managed resources, and critically reviewed the manuscript.
Corresponding authors
Ethics declarations
All procedures related to animal studies were approved by the institutional animal committee at UTHSC in full accordance with the ethical guidelines of the National Institutes of Health for the care and use of laboratory animals.
Consent to Participate
Not applicable
Consent for Publication
All authors have agreed to publish this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salman, M., Stayton, A.S., Parveen, K. et al. Intranasal Delivery of Mitochondria Attenuates Brain Injury by AMPK and SIRT1/PGC-1α Pathways in a Murine Model of Photothrombotic Stroke. Mol Neurobiol 61, 2822–2838 (2024). https://doi.org/10.1007/s12035-023-03739-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03739-4