Abstract
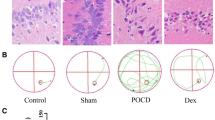
The present work aimed to explore the role of long non-coding RNA (lncRNA)-AC020978 in postoperative cognitive disorder (POCD) and the underlying mechanism. The POCD mouse model was constructed through isoflurane anesthesia + abbreviated laparotomy. The AC020978 expression in brain tissue was silenced after lentivirus injection, then Morris water maze test was conducted to detect the cognitive disorder level, flow cytometry was performed to analyze M1 macrophage level, ELISA was carried out to measure inflammatory factor levels, H&E, Nissl and immunohistochemical staining was performed to detect the pathological changes in brain tissue, and Western blotting assay was adopted to detect protein expression. In addition, microglial cells were cultured in vitro, after lentivirus infection, the effect of AC020978 on the M1 polarization of microglial cells and glycolysis was observed. AC020978 overexpression promoted POCD progression and aggravated cognitive disorder in mice; in addition, the proportion of peripheral and central M1 cells increased, the inflammatory factor levels were upregulated, and microglial cells were activated. By contrast, AC020978 silencing led to cognitive disorder in mice and suppressed microglial cell activation and M1 polarization. In vitro experimental results indicated that AC020978 promoted the expression and phosphorylation of PKM2, which promoted inflammatory response through enhancing microglial cell glycolysis and M1 polarization. AC020978 interacts with PKM2 to promote the glycolysis and M1 polarization of microglial cells, thus regulating cognitive disorder and central inflammation in POCD.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X (2020) The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol 130:110791
Yang Y, Liu Y, Zhu J, Song S, Huang Y, Zhang W, Sun Y, Hao J et al (2022) Neuroinflammation-mediated mitochondrial dysregulation involved in postoperative cognitive dysfunction. Free Radic Biol Med 178:134–146
Xie X, Shen Z, Hu C, Zhang K, Guo M, Wang F, Qin K (2021) Dexmedetomidine ameliorates postoperative cognitive dysfunction in aged mice. Neurochem Res 46(9):2415–2426
Granger KT, Barnett JH (2021) Postoperative cognitive dysfunction: an acute approach for the development of novel treatments for neuroinflammation. Drug Discov Today 26(5):1111–1114
Han X, Cheng X, Xu J, Liu Y, Zhou J, Jiang L, Gu X, Xia T (2022) Activation of TREM2 attenuates neuroinflammation via PI3K/Akt signaling pathway to improve postoperative cognitive dysfunction in mice. Neuropharmacology 219:109231
Guéniot L, Lepere V, De Medeiros GF, Danckaert A, Flamant P, Le Dudal M, Langeron O, Goossens PL et al (2020) Muscle injury induces postoperative cognitive dysfunction. Sci Rep 10(1):2768
Yu K, Zhang XK, Xiong HC, Liang SS, Lu ZY, Wu YQ, Chen Y, Xiao SJ (2023) Stellate ganglion block alleviates postoperative cognitive dysfunction via inhibiting TLR4/NF-κB signaling pathway. Neurosci Lett 807:137259
Tan X, Wang J, Yao J, Yuan J, Dai Y, Sun M, Zhang T, Yang J et al (2023) Microglia participate in postoperative cognitive dysfunction by mediating the loss of inhibitory synapse through the complement pathway. Neurosci Lett 796:137049
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496(7446):445–455
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D (2020) Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol 11:1731
Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J, Huang G (2020) Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1α axis. Theranostics 10(11):4762–4778
van Zuylen ML, Gribnau A, Admiraal M, Ten Hoope W, Veelo DP, Hollmann MW, Preckel B, Hermanides J (2021) The role of intraoperative hypotension on the development of postoperative cognitive dysfunction: a systematic review. J Clin Anesth 72:110310
Tan XX, Qiu LL, Sun J (2021) Research progress on the role of inflammatory mechanisms in the development of postoperative cognitive dysfunction. Biomed Res Int 2021:3883204
Hua M, Min J (2020) Postoperative cognitive dysfunction and the protective effects of enriched environment: a systematic review. Neurodegener Dis 20(4):113–122
Chen S, Zhang Y, Dai W, Qi S, Tian W, Gu X, Chen X, Yu W et al (2020) Publication trends and hot spots in postoperative cognitive dysfunction research: a 20-year bibliometric analysis. J Clin Anesth 67:110012
Subramaniyan S, Terrando N (2019) Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg 128(4):781–788
Hongyu X, Qingting W, Xiaoling S, Liwu Z, Ailing Y, Xin L (2019) Penehyclidine hydrochloride on postoperatively cognitive function. Med Hypotheses 129:109246
Voet S, Prinz M, van Loo G (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 25(2):112–123
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42
Subhramanyam CS, Wang C, Hu Q, Dheen ST (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120
Meng F, Yu W, Duan W, Wang T, Liu Y (2020) Dexmedetomidine attenuates LPS-mediated BV2 microglia cells inflammation via inhibition of glycolysis. Fundam Clin Pharmacol 34(3):313–320
Wang L, Pavlou S, Du X, Bhuckory M, Xu H, Chen M (2019) Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener 14(1):2
Yan Y, Lv Q, Zhou F, Jian Y, Xinhua L, Chen X, Hu Y (2023) Discovery of an effective anti-inflammatory agent for inhibiting the activation of NF-κB. J Enzyme Inhib Med Chem 38(1):2225135
Acknowledgements
I need to thank my colleagues and friends for their help and support in the research, Thanks for the support of the Science and technology planning project of JiaXing.
Funding
The Science and technology planning project of JiaXing [2023AZ11001].
Author information
Authors and Affiliations
Contributions
Genghuan Wang and Jian Shen are responsible for the experiment and acquisition of relevant data; Qiaobing Guan and Yingcong Lin are responsible for paper writing; Liping Zhai and Heping Shen are responsible for project development, financial support.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to Participate
The study was approved with Ethics Committee.
Consent for Publication
All authors approved to publish the article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Shen, J., Guan, Q. et al. LncRNA-AC020978 Promotes Metabolic Reprogramming in M1 Microglial Cells in Postoperative Cognitive Disorder via PKM2. Mol Neurobiol 61, 2459–2467 (2024). https://doi.org/10.1007/s12035-023-03729-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03729-6