Abstract
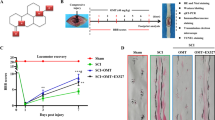
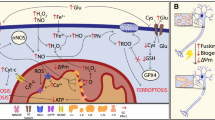
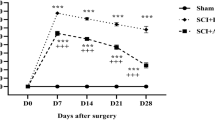
The key role of mitochondria in neurodegenerative disease patients is well documented. Recent studies claimed that mitochondrial regulatory dysfunction might play a role in ongoing cell death and dysfunction. In the present study, we characterized ultrastructural morphometry of mitochondrial alterations occurring at the level of motor neuron cell bodies in SCI-induced rats. We applied 17β-estradiol (E2) to determine whether it can improve mitochondria structural integrity of motor neurons. We used a rat model of acute SCI generated by spinal cord contusion at the T9–T10 level, followed by tissue processing 21 days post-SCI. Samples were divided into five groups: laminectomy, SCI, vehicle, SCI + 25 µg/kg E2, and SCI + 10 µg/kg E2. Assessments included analysis of hind limb motor recovery, quantifying tissue repair, and evaluation of morphological changes in the ultrastructure of mitochondria in motor neurons by transmission electron microscopy. In the E2-treated groups, especially the group receiving 25 µg/kg E2, less irregular mitochondria were observed, as there was a significant reduction in swelling or vacuolization, or fragmentation compared to the SCI group. Furthermore, E2 significantly reduced membrane rupture in the SCI group. E2 could be a proper therapeutic agent to relieve mitochondrial deleterious effects on neurons in neurodegenerative diseases.




Similar content being viewed by others
Data Availability
Research data are not shared for this study.
Abbreviations
- SCI:
-
Spinal cord injury
- E2:
-
17β-Estradiol
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- BBB:
-
Basso, Beattie, and Bresnahan
- TEM:
-
Transmission electron microscopy
- LFB:
-
Luxol Fast Blue
- PBS:
-
Phosphate-buffered saline
- Ca2+ :
-
Calcium ions
- ATP:
-
Adenosine triphosphate
References
Namjoo Z, Moradi F, Aryanpour R, Piryaei A, Joghataei MT, Abbasi Y, Hosseini A, Hassanzadeh S et al (2018) Combined effects of rat Schwann cells and 17β-estradiol in a spinal cord injury model. Metab Brain Dis 33:1229–1242. https://doi.org/10.1007/s11011-018-0220-8
Griffin JM, Bradke F (2020) Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol Med 12:e11505. https://doi.org/10.15252/emmm.201911505
Hassanzadeh S, Jameie SB, Mehdizadeh M, Soleimani M, Namjoo Z, Soleimani M (2018) FNDC5 expression in Purkinje neurons of adult male rats with acute spinal cord injury following treatment with methylprednisolone. Neuropeptides 70:16–25. https://doi.org/10.1016/j.npep.2018.05.002
Fuchs A, Kutterer S, Mühling T, Duda J, Schütz B, Liss B, Keller BU, Roeper J (2013) Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. J Physiol 591:2723–2745. https://doi.org/10.1113/jphysiol.2012.247981
Barrett EF, Barrett JN, David G (2014) Dysfunctional mitochondrial Ca(2+) handling in mutant SOD1 mouse models of fALS: integration of findings from motor neuron somata and motor terminals. Front Cell Neurosci 8:184. https://doi.org/10.3389/fncel.2014.00184
Kodavati M, Wang H, Hegde ML (2020) Altered mitochondrial dynamics in motor neuron disease: an emerging perspective. Cells 9. https://doi.org/10.3390/cells9041065
Green A, Hossain T, Eckmann DM (2022) Mitochondrial dynamics involves molecular and mechanical events in motility, fusion and fission. Front Cell Dev Biol 10:1010232. https://doi.org/10.3389/fcell.2022.1010232
Beckervordersandforth R, Ebert B, Schäffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L et al (2017) Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron 93:560-573.e6. https://doi.org/10.1016/j.neuron.2016.12.017
Fernandez-Fernandez S, Almeida A, Bolaños JP (2012) Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J 443:3–11. https://doi.org/10.1042/bj20111943
Arnold S, de Araújo GW, Beyer C (2008) Gender-specific regulation of mitochondrial fusion and fission gene transcription and viability of cortical astrocytes by steroid hormones. J Mol Endocrinol 41:289–300. https://doi.org/10.1677/jme-08-0085
Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280:26185–26192. https://doi.org/10.1074/jbc.M503062200
Jiao L, Wang J, Zhao W, Zhu X, Meng X, Zhao L (2020) Comparison of the effect of 1-day and 2-day low residue diets on the quality of bowel preparation before colonoscopy. Saudi J Gastroenterol 26:137–143. https://doi.org/10.4103/sjg.SJG_471_19
McEwen ML, Sullivan PG, Rabchevsky AG, Springer JE (2011) Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8:168–179. https://doi.org/10.1007/s13311-011-0031-7
Rabchevsky AG, Michael FM, Patel SP (2020) Mitochondria focused neurotherapeutics for spinal cord injury. Exp Neurol 330:113332. https://doi.org/10.1016/j.expneurol.2020.113332
Karbowski M, Youle RJ (2003) Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 10:870–880. https://doi.org/10.1038/sj.cdd.4401260
Holtz A, Nyström B, Gerdin B (1989) Blocking weight-induced spinal cord injury in rats: effects of TRH or naloxone on motor function recovery and spinal cord blood flow. Acta Neurol Scand 80:215–220. https://doi.org/10.1111/j.1600-0404.1989.tb03865.x
Zendedel A, Mönnink F, Hassanzadeh G, Zaminy A, Ansar MM, Habib P, Slowik A, Kipp M, Beyer C (2018) Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol 55:1364–1375. https://doi.org/10.1007/s12035-017-0400-2
Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL (2016) Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J Neurochem 137:604–617. https://doi.org/10.1111/jnc.13610
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21. https://doi.org/10.1089/neu.1995.12.1
Namjoo Z, Mortezaee K, Joghataei MT, Moradi F, Piryaei A, Abbasi Y, Hosseini A, Majidpoor J (2018) Targeting axonal degeneration and demyelination using combination administration of 17β-estradiol and Schwann cells in the rat model of spinal cord injury. J Cell Biochem 119:10195–10203. https://doi.org/10.1002/jcb.27361
Nazari B, Namjoo Z, Moradi F, Kazemi M, Ebrahimi-Barough S, Sadroddiny E, Ai J (2021) miR-219 overexpressing oligodendrocyte progenitor cells for treating compression spinal cord injury. Metab Brain Dis 36:1069–1077. https://doi.org/10.1007/s11011-021-00701-y
Natale G, Lenzi P, Lazzeri G, Falleni A, Biagioni F, Ryskalin L, Fornai F (2015) Compartment-dependent mitochondrial alterations in experimental ALS, the effects of mitophagy and mitochondriogenesis. Front Cell Neurosci 9:434. https://doi.org/10.3389/fncel.2015.00434
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023. https://doi.org/10.1523/jneurosci.21-09-03017.2001
Granatiero V, Manfredi G (2019) Mitochondrial transport and turnover in the pathogenesis of amyotrophic lateral sclerosis. Biology (Basel) 8. https://doi.org/10.3390/biology8020036
Gao Z, Pang Z, Chen Y, Lei G, Zhu S, Li G, Shen Y, Xu W (2022) Restoring after central nervous system injuries: neural mechanisms and translational applications of motor recovery. Neurosci Bull 38:1569–1587. https://doi.org/10.1007/s12264-022-00959-x
Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL (2010) Effect of endogenous androgens on 17beta-estradiol-mediated protection after spinal cord injury in male rats. J Neurotrauma 27:611–626. https://doi.org/10.1089/neu.2009.1069
Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW (2005) NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain 128:839–853. https://doi.org/10.1093/brain/awh424
Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH, Li H (2000) A mouse model for spinal muscular atrophy. Nat Genet 24:66–70. https://doi.org/10.1038/71709
Miller N, Shi H, Zelikovich AS, Ma YC (2016) Motor neuron mitochondrial dysfunction in spinal muscular atrophy. Hum Mol Genet 25:3395–3406. https://doi.org/10.1093/hmg/ddw262
Gonzalez Deniselle MC, Lopez Costa JJ, Gonzalez SL, Labombarda F, Garay L, Guennoun R, Schumacher M, De Nicola AF (2002) Basis of progesterone protection in spinal cord neurodegeneration. J Steroid Biochem Mol Biol 83:199–209. https://doi.org/10.1016/S0960-0760(02)00262-5
Aryanpour R, Zibara K, Pasbakhsh P, Jame’ei SB, Namjoo Z, Ghanbari A, Mahmoudi R, Amani S et al (2021) 17β-Estradiol reduces demyelination in cuprizone-fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience 463:116–127. https://doi.org/10.1016/j.neuroscience.2021.03.025
Zhao J, Wang X, Huo Z, Chen Y, Liu J, Zhao Z, Meng F, Su Q, et al (2022) The impact of mitochondrial dysfunction in amyotrophic lateral sclerosis. Cells 11. https://doi.org/10.3390/cells11132049
Acknowledgements
The authors would like to appreciate Dr. Mohsen Sagha for formatting of the figures and for the additional comments.
Author information
Authors and Affiliations
Contributions
Zeinab Namjoo conceived the experimental design and provided supervision during the research; all the authors performed experimental work and wrote the article.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Research Ethics Committees of Ardabil University of Medical Sciences, Ardabil, Iran (IR.IAU.TABRIZ.REC.1401.056).
Consent to Publication
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2023_3710_MOESM1_ESM.tif
Supplementary file1. TEM imaging. Electron micrographs revealed robust ultrastructural differences in motor neural mitochondria between laminectomy, Vehicle, 10µg/kg E2 and 25µg/kg E2 groups (TIF 2683 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassanzadeh, S., Sabetvand, M., Sardar, R. et al. Spinal Cord Injury Model Mitochondria Connect Altered Function with Defects of Mitochondrion Morphology: an Ultrastructural Study. Mol Neurobiol 61, 2241–2248 (2024). https://doi.org/10.1007/s12035-023-03710-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03710-3