Abstract
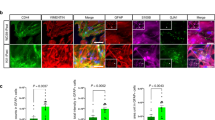
Mitochondrial dysfunction is one of the hallmarks in the pathophysiology of prion disease and other neurodegenerative diseases. Various metabolic dysfunctions are identified and considered to contribute to the progression of some types of neurodegenerative diseases. In this study, we evaluated the status of glycolysis pathway in prion-infected rodent and cell models. The levels of the key enzymes, hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PK) were significantly increased, accompanying with markedly downregulated mitochondrial complexes. Double-stained IFAs revealed that the increased HK2 and PFK distributed widely in GFAP-, Iba1-, and NeuN-positive cells. We also identified increased levels of AMP-activated protein kinase (AMPK) and the downstream signaling. Changes of AMPK activity in prion-infected cells by the AMPK-specific inhibitor or activator induced the corresponding alterations not only in the downstream signaling, but also the expressions of three key kinases in glycolysis pathway and the mitochondrial complexes. Transient removal or complete clearance of prion propagation in the prion-infected cells partially but significantly reversed the increases of the key enzymes in glycolysis, the upregulation of AMPK signaling pathway, and the decreases of the mitochondrial complexes. Measurements of the cellular oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) showed lower OCR and higher ECAR in prion-infected cell line, which were sufficiently reversed by clearance of prion propagation. Those data indicate a metabolic reprogramming from oxidative phosphorylation to glycolysis in the brains during the progression of prion disease. Accumulation of PrPSc is critical for the switch to glycolysis, largely via activating AMPK pathway.



















Similar content being viewed by others
Data Availability
The datasets generated during and analyzed during the current study are not publicly available due to data security but are available from the corresponding author on reasonable request.
References
Chen C, Dong XP (2016) Epidemiological characteristics of human prion diseases. Infect Dis Poverty 5(1):47. https://doi.org/10.1186/s40249-016-0143-8
Shi Q, Chen C, Xiao K, Zhou W, Gao C, Gao L et al (2022) Extensive disturbances of intracellular components and dysfunctions of biological pathways in the brain tissues during prion infection—China’s studies. China CDC Weekly 4(10):741–747. https://doi.org/10.46234/ccdcw2022.154
Zhu T, Chen JL, Wang Q, Shao W, Qi B (2018) Modulation of mitochondrial dynamics in neurodegenerative diseases: an insight into prion diseases. Front Aging Neurosci 10:336. https://doi.org/10.3389/fnagi.2018.00336
Beard E, Lengacher S, Dias S, Magistretti PJ, Finsterwald C (2021) Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front Physiol 12:825816. https://doi.org/10.3389/fphys.2021.825816
Demetrius LA, Driver JA (2015) Preventing Alzheimer's disease by means of natural selection. J R Soc Interface 12(102):20140919. https://doi.org/10.1098/rsif.2014.0919
Demetrius LA, Magistretti PJ, Pellerin L (2014) Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect. Front Physiol 5:522. https://doi.org/10.3389/fphys.2014.00522
Li XB, Gu JD, Zhou QH (2015) Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac Cancer 6(1):17–24. https://doi.org/10.1111/1759-7714.12148
Bell SM, Burgess T, Lee J, Blackburn DJ, Allen SP, Mortiboys H (2020) Peripheral glycolysis in neurodegenerative diseases. Int J Mol Sci 21(23). https://doi.org/10.3390/ijms21238924
Bigl M, Bleyl AD, Zedlick D, Arendt T, Bigl V, Eschrich K (1996) Changes of activity and isozyme pattern of phosphofructokinase in the brains of patients with Alzheimer's disease. J Neurochem 67(3):1164–1171. https://doi.org/10.1046/j.1471-4159.1996.67031164.x
Sonntag KC, Ryu WI, Amirault KM, Healy RA, Siegel AJ, McPhie DL et al (2017) Late-onset Alzheimer's disease is associated with inherent changes in bioenergetics profiles. Sci Rep 7(1):14038. https://doi.org/10.1038/s41598-017-14420-x
Yan X, Hu Y, Wang B, Wang S, Zhang X (2020) Metabolic dysregulation contributes to the progression of Alzheimer's disease. Front Neurosci 14:530219. https://doi.org/10.3389/fnins.2020.530219
Xiao K, Zhang BY, Zhang XM, Wang J, Chen C, Chen LN et al (2016) Re-infection of the prion from the scrapieinfected cell line SMB-S15 in three strains of mice, CD1, C57BL/6 and Balb/c. Int J Mol Med 37(3):716–726. https://doi.org/10.3892/ijmm.2016.2465
Shi Q, Zhang BY, Gao C, Zhang J, Jiang HY, Chen C et al (2012) Mouse-adapted scrapie strains 139A and ME7 overcome species barrier to induce experimental scrapie in hamsters and changed their pathogenic features. Virol J 9:63. https://doi.org/10.1186/1743-422X-9-63
Chen C, Xu XF, Zhang RQ, Ma Y, Lv Y, Li JL et al (2017) Remarkable increases of α1-antichymotrypsin in brain tissues of rodents during prion infection. Prion 11(5):338–351. https://doi.org/10.1080/19336896.2017.1349590
Clarke MC, Haig DA (1971) Multiplication of scrapie agent in mouse spleen. Res Vet Sci 12(2):195–197
Birkett CR, Hennion RM, Bembridge DA, Clarke MC, Chree A, Bruce ME et al (2001) Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J 20(13):3351–3358. https://doi.org/10.1093/emboj/20.13.3351
Wang J, Zhang BY, Zhang J, Xiao K, Chen LN, Wang H et al (2016) Treatment of SMB-S15 Cells with resveratrol efficiently removes the PrP(Sc) accumulation in vitro and prion infectivity in vivo. Mol Neurobiol 53(8):5367–5376. https://doi.org/10.1007/s12035-015-9464-z
Ma Y, Shi Q, Wang J, Xiao K, Sun J, Lv Y et al (2017) Reduction of NF-κB (p65) in scrapie-infected cultured cells and in the brains of scrapie-infected rodents. ACS Chem Neurosci 8(11):2535–2548. https://doi.org/10.1021/acschemneuro.7b00273
Illarioshkin SN, Klyushnikov SA, Vigont VA, Seliverstov YA, Kaznacheyeva EV. Molecular pathogenesis in Huntington's disease. 1608-3040 (Electronic)
Singh AA-O, Kukreti R, Saso LA-O, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. https://doi.org/10.3390/molecules24081583
Cai QA-O, Jeong YY. Mitophagy in Alzheimer's disease and other age-related neurodegenerative diseases. https://doi.org/10.3390/cells9010150
Monzio Compagnoni GA-O, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E. The role of mitochondria in neurodegenerative diseases: the lesson from Alzheimer's disease and Parkinson's disease. 1559-1182 (Electronic)
Butterfield DA, Boyd-Kimball D. Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer's disease. 1875-8908 (Electronic)
Harley J, Clarke BE, Patani RA-O The interplay of RNA binding proteins, oxidative stress and mitochondrial dysfunction in ALS. https://doi.org/10.3390/antiox10040552
Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem 109(Suppl 1):153–159. https://doi.org/10.1111/j.1471-4159.2009.05867.x
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta 1842(8):1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Wang W, Zhao F, Ma X, Perry G, Zhu X (2020) Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener 15(1):30. https://doi.org/10.1186/s13024-020-00376-6
Powers WJ, Videen TO, Markham J, McGee-Minnich L, Antenor-Dorsey JV, Hershey T et al (2007) Selective defect of in vivo glycolysis in early Huntington's disease striatum. Proc Natl Acad Sci U S A 104(8):2945–2949. https://doi.org/10.1073/pnas.0609833104
Shah SZA, Zhao D, Hussain T, Sabir N, Mangi MH, Yang L (2018) p62-Keap1-NRF2-ARE pathway: a contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front Mol Neurosci 11:310. https://doi.org/10.3389/fnmol.2018.00310
Li C, Wang D, Wu W, Yang W, Ali Shah SZ, Zhao Y et al (2018) DLP1-dependent mitochondrial fragmentation and redistribution mediate prion-associated mitochondrial dysfunction and neuronal death. Aging Cell 17(1). https://doi.org/10.1111/acel.12693
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583-019-0132-6
Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB et al (2020) Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov 19(9):609–633. https://doi.org/10.1038/s41573-020-0072-x
Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI et al (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer's disease. Cell Metab 30(3):493–507 e6. https://doi.org/10.1016/j.cmet.2019.06.005
Choi H, Choi Y, Lee EJ, Kim H, Lee Y, Kwon S et al (2021) Hippocampal glucose uptake as a surrogate of metabolic change of microglia in Alzheimer's disease. J Neuroinflammation 18(1):190. https://doi.org/10.1186/s12974-021-02244-6
Sameni S, Syed A, Marsh JL, Digman MA (2016) The phasor-FLIM fingerprints reveal shifts from OXPHOS to enhanced glycolysis in Huntington disease. Sci Rep 6:34755. https://doi.org/10.1038/srep34755
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135. https://doi.org/10.1038/nrm.2017.95
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL et al (2017) TREM2 maintains microglial metabolic fitness in Alzheimer's disease. Cell 170(4):649–63.e13. https://doi.org/10.1016/j.cell.2017.07.023
Ciccarese F, Zulato E, Indraccolo S (2019) LKB1/AMPK pathway and drug response in cancer: a therapeutic perspective. Oxid Med Cell Longev 2019:8730816. https://doi.org/10.1155/2019/8730816
Garcia D, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66(6):789–800. https://doi.org/10.1016/j.molcel.2017.05.032
Zhang S, Lachance BB, Mattson MP, Jia X (2021) Glucose metabolic crosstalk and regulation in brain function and diseases. Prog Neurobiol 204:102089. https://doi.org/10.1016/j.pneurobio.2021.102089
Moon JH, Park SY (2020) Prion peptide-mediated calcium level alteration governs neuronal cell damage through AMPK-autophagy flux. Cell Commun Signal 18(1):109. https://doi.org/10.1186/s12964-020-00590-1
Fan XY, Tian C, Wang H, Xu Y, Ren K, Zhang BY et al (2015) Activation of the AMPK-ULK1 pathway plays an important role in autophagy during prion infection. Sci Rep 5:14728. https://doi.org/10.1038/srep14728
Traxler L, Herdy JR, Stefanoni D, Eichhorner S, Pelucchi S, Szücs A et al (2022) Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer's disease. Cell Metab 34(9):1248–63.e6. https://doi.org/10.1016/j.cmet.2022.07.014
Cheng J, Zhang R, Xu Z, Ke Y, Sun R, Yang H et al (2021) Early glycolytic reprogramming controls microglial inflammatory activation. J Neuroinflammation 18(1):129. https://doi.org/10.1186/s12974-021-02187-y
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Vaupel P, Multhoff G (2021) Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 599(6):1745–1757. https://doi.org/10.1113/jp278810
Zong WX, Rabinowitz JD, White E (2016) Mitochondria and cancer. Mol Cell 61(5):667–676. https://doi.org/10.1016/j.molcel.2016.02.011
Lu J, Tan M, Cai Q (2015) The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356(2 Pt A):156–164. https://doi.org/10.1016/j.canlet.2014.04.001
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337. https://doi.org/10.1038/nrc3038
Min J, Zeng T, Roux M, Lazar D, Chen L, Tudzarova S (2021) The role of HIF1α-PFKFB3 pathway in diabetic retinopathy. J Clin Endocrinol Metab 106(9):2505–2519. https://doi.org/10.1210/clinem/dgab362
Abedpoor N, Taghian F, Hajibabaie F (2022) Cross brain-gut analysis highlighted hub genes and LncRNA networks differentially modified during leucine consumption and endurance exercise in mice with depression-like behaviors. Mol Neurobiol 59(7):4106–4123. https://doi.org/10.1007/s12035-022-02835-1
Hajibabaie F, Abedpoor N, Taghian F, Safavi K (2023) A cocktail of polyherbal bioactive compounds and regular mobility training as senolytic approaches in age-dependent Alzheimer's: the in silico analysis, lifestyle intervention in old age. J Mol Neurosci 73(2-3):171–184. https://doi.org/10.1007/s12031-022-02086-8
Gao C, Wei J, Zhang BY, Shi Q, Chen C, Wang J et al (2016) MiRNA expression profiles in the brains of mice infected with scrapie agents 139A, ME7 and S15. Emerg Microbes Infect 5(11):e115. https://doi.org/10.1038/emi.2016.120
Cheng L, Quek C, Li X, Bellingham SA, Ellett LJ, Shambrook M et al (2021) Distribution of microRNA profiles in pre-clinical and clinical forms of murine and human prion disease. Commun Biol 4(1):411. https://doi.org/10.1038/s42003-021-01868-x PubMed PMID: 33767334; PubMed Central PMCID: PMCPMC7994852 and A.F.H. report grants from the National Health and Medical Research Council of Australia, grants from the Australian Creutzfeldt-Jakob Disease Support Group Network, during the conduct of the study; In addition, L.C. and A.F.H. have patented novel methods for dementia diagnosis (Provisional patent #: 2018904605) pending. A.F.H. is a shareholder in D-Gen Ltd which markets the ICSM18 antibody used in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Slota JA, Booth SA (2019) MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA 5(2). https://doi.org/10.3390/ncrna5020035
Pease D, Scheckel C, Schaper E, Eckhardt V, Emmenegger M, Xenarios I et al (2019) Genome-wide identification of microRNAs regulating the human prion protein. Brain Pathol 29(2):232–244. https://doi.org/10.1111/bpa.12679
Funding
This work was supported by the Chinese National Natural Science Foundation Grants (81630062) and Grant (2019SKLID501, 2019SKLID603, 2019SKLID307) from the State Key Laboratory for Infectious Disease Prevention and Control, China CDC.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The preparations of materials and the performances of the experiments were conducted by FQ, WYZ, CDD, HC, LCM, LX, and ZWW. Data collection and analysis were conducted by FQ, XK, GLP, JXX, and CC. FQ, SQ, and DXP drafted the manuscript. SQ and XPD contributed to design, study concept, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Chinese Laboratory Animal Management. Approval was granted by the Research Ethics Committee of the National Institute for Viral Disease Control and Prevention, China CDC.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication of the images in figures.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Q., Xiao, K., A, R. et al. Accumulation of Prion Triggers the Enhanced Glycolysis via Activation of AMKP Pathway in Prion-Infected Rodent and Cell Models. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03621-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03621-3