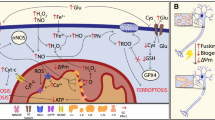
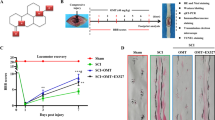
Abstract
Mitochondria are important organelle of eukaryotic cells. They consists of a large number of different proteins that provide most of the ATP and supply power for the growth, function, and regeneration of neurons. Therefore, smitochondrial transport ensures that adequate ATP is supplied for metabolic activities. Spinal cord injury (SCI), a detrimental condition, has high morbidity and mortality rates. Currently, the available treatments only provide symptomatic relief for long-term disabilities. Studies have implicated mitochondrial transport as a critical factor in axonal regeneration. Hence, enhancing mitochondrial transports could be beneficial for ameliorating SCI. Syntaphilin (Snph) is a mitochondrial docking protein that acts as a “static anchor,” and its inhibition enhances mitochondrial transports. Therefore, Snph as a key mediator of mitochondrial transports, may contribute to improving axonal regeneration following SCI. Herein, we examine Snph’s biological effects and its relation to mitochondrial pathway. Then, we elaborate on mitochondrial transports after SCI, the possible role of Snph in SCI, and some possible therapeutic approaches by Snph.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Jin LY, Li J, Wang KF, Xia WW, Zhu ZQ, Wang CR, Li XF, Liu HY (2021) Blood-spinal cord barrier in spinal cord injury: a review. J Neurotrauma 38(9):1203–1224. https://doi.org/10.1089/neu.2020.7413
Katoh H, Yokota K, Fehlings MG (2019) Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci 13:248. https://doi.org/10.3389/fncel.2019.00248
Lu Q, Botchway BOA, Zhang Y, Jin T, Liu X (2022) SARM1 can be a potential therapeutic target for spinal cord injury. Cell Mol Life Sci 79(3):161. https://doi.org/10.1007/s00018-022-04195-4
Hayta E, Elden H (2018) Acute spinal cord injury: a review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat 87:25–31. https://doi.org/10.1016/j.jchemneu.2017.08.001
Wang XJ, Peng CH, Zhang S, Xu XL, Shu GF, Qi J, Zhu YF, Xu DM et al (2019) Polysialic-acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett 19(2):829–838. https://doi.org/10.1021/acs.nanolett.8b04020
Allison DJ, Thomas A, Beaudry K, Ditor DS (2016) Targeting inflammation as a treatment modality for neuropathic pain in spinal cord injury: a randomized clinical trial. J Neuroinflammation 13(1):152. https://doi.org/10.1186/s12974-016-0625-4.PMID:27316678;PMCID:PMC4912827
Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S et al (2016) Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports 6(1):1–8. https://doi.org/10.1016/j.stemcr.2015.11.013
Xue F, Wu EJ, Zhang PX, Li-Ya A, Kou YH, Yin XF, Han N (2015) Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen Res 10(1):104–111. https://doi.org/10.4103/1673-5374.150715
Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C et al (2015) Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348(6232):347–352. https://doi.org/10.1126/science.aaa2958
Tse CM, Chisholm AE, Lam T, Eng JJ, SCIRE Research Team (2018) A systematic review of the effectiveness of task-specific rehabilitation interventions for improving independent sitting and standing function in spinal cord injury. J Spinal Cord Med 41(3):254–266. https://doi.org/10.1080/10790268.2017.1350340
Han Q, Xie Y, Ordaz JD, Huh AJ, Huang N, Wu W, Liu N, Chamberlain KA, et al. (2020) Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 31(3):623–641.e8. https://doi.org/10.1016/j.cmet.2020.02.002
Petrova V, Nieuwenhuis B, Fawcett JW, Eva R (2021) Axonal organelles as molecular platforms for axon growth and regeneration after injury. Int J Mol Sci 22(4):1798. https://doi.org/10.3390/ijms22041798
He Z, Jin Y (2016) Intrinsic control of axon regeneration. Neuron 90(3):437–451. https://doi.org/10.1016/j.neuron.2016.04.022
Petrova V, Eva R (2018) The virtuous cycle of axon growth: axonal transport of growth-promoting machinery as an intrinsic determinant of axon regeneration. Dev Neurobiol 78(10):898–925. https://doi.org/10.1002/dneu.22608
Scholpa NE, Schnellmann RG (2017) Mitochondrial-based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J Pharmacol Exp Ther 363(3):303–313. https://doi.org/10.1124/jpet.117.244806
Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH (2016) Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol 214(1):103–119. https://doi.org/10.1083/jcb.201605101
Stefanatos R, Sanz A (2018) The role of mitochondrial ROS in the aging brain. FEBS Lett 592(5):743–758. https://doi.org/10.1002/1873-3468.12902
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. https://doi.org/10.1038/nature18928
Lightowlers RN, Chrzanowska-Lightowlers ZM, Russell OM (2020) Mitochondrial transplantation-a possible therapeutic for mitochondrial dysfunction?: mitochondrial transfer is a potential cure for many diseases but proof of efficacy and safety is still lacking. EMBO Rep 21(9):e50964. https://doi.org/10.15252/embr.202050964
Huang N, Li S, Xie Y, Han Q, Xu XM, Sheng ZH (2021) Reprogramming an energetic AKT-PAK5 axis boosts axon energy supply and facilitates neuron survival and regeneration after injury and ischemia. Curr Biol 31(14):3098-3114.e7. https://doi.org/10.1016/j.cub.2021.04.079
Miki A, Kanamori A, Nakamura M, Matsumoto Y, Mizokami J, Negi A (2014) The expression of syntaphilin is down-regulated in the optic nerve after axonal injury. Exp Eye Res 129:38–47. https://doi.org/10.1016/j.exer.2014.10.017
Hwang MJ, Bryant KG, Seo JH, Liu Q, Humphrey PA, Melnick MAC, Altieri DC, Robert ME (2019) Syntaphilin is a novel biphasic biomarker of aggressive prostate cancer and a metastasis predictor. Am J Pathol 189(6):1180–1189. https://doi.org/10.1016/j.ajpath.2019.02.009
Seo JH, Agarwal E, Bryant KG, Caino MC, Kim ET, Kossenkov AV, Tang HY, Languino LR et al (2018) Syntaphilin ubiquitination regulates mitochondrial dynamics and tumor cell movements. Cancer Res 78(15):4215–4228. https://doi.org/10.1158/0008-5472.CAN-18-0595
Chen X, Xu W, Zhuo S, Chen X, Chen P, Guan S, Huang D, Sun X et al (2021) Syntaphilin downregulation facilitates radioresistance via mediating mitochondria distribution in esophageal squamous cell carcinoma. Free Radic Biol Med 165:348–359. https://doi.org/10.1016/j.freeradbiomed.2021.01.056
Lin MY, Cheng XT, Xie Y, Cai Q, Sheng ZH (2017) Removing dysfunctional mitochondria from axons independent of mitophagy under pathophysiological conditions. Autophagy 13(10):1792–1794. https://doi.org/10.1080/15548627.2017.1356552
Feng Y, Guo M, Zhao H, Han S, Hao Y, Yuan Y, Shen W, Sun J et al (2021) Dl-3-n-Butylphthalide alleviates demyelination and improves cognitive function by promoting mitochondrial dynamics in white matter lesions. Front Aging Neurosci 13:632374. https://doi.org/10.3389/fnagi.2021.632374
Caino MC, Seo JH, Aguinaldo A, Wait E, Bryant KG, Kossenkov AV, Hayden JE, Vaira V et al (2016) A neuronal network of mitochondrial dynamics regulates metastasis. Nat Commun 7:13730. https://doi.org/10.1038/ncomms13730
Chen YM, Gerwin C, Sheng ZH (2009) Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J Neurosci 29(30):9429–9438. https://doi.org/10.1523/JNEUROSCI.1472-09.2009
Lao G, Scheuss V, Gerwin CM, Su Q, Mochida S, Rettig J, Sheng ZH (2000) Syntaphilin: a syntaxin-1 clamp that controls SNARE assembly. Neuron 25(1):191–201. https://doi.org/10.1016/s0896-6273(00)80882-x
Li S, Xiong GJ, Huang N, Sheng ZH (2020) The cross-talk of energy sensing and mitochondrial anchoring sustains synaptic efficacy by maintaining presynaptic metabolism. Nat Metab 2(10):1077–1095. https://doi.org/10.1038/s42255-020-00289-0
Cheng XT, Huang N, Sheng ZH (2022) Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron 110(12):1899–1923. https://doi.org/10.1016/j.neuron.2022.03.015
Cheng XT, Sheng ZH (2021) Developmental regulation of microtubule-based trafficking and anchoring of axonal mitochondria in health and diseases. Dev Neurobiol 81(3):284–299. https://doi.org/10.1002/dneu.22748
Lin MY, Sheng ZH (2015) Regulation of mitochondrial transport in neurons. Exp Cell Res 334(1):35–44. https://doi.org/10.1016/j.yexcr.2015.01.004
Zheng Y, Zhang X, Wu X, Jiang L, Ahsan A, Ma S, Xiao Z, Han F et al (2019) Somatic autophagy of axonal mitochondria in ischemic neurons. J Cell Biol 218(6):1891–1907. https://doi.org/10.1083/jcb.201804101
Shi X, Jiang X, Chen C, Zhang Y, Sun X (2022) The interconnections between the microtubules and mitochondrial networks in cardiocerebrovascular diseases: implications for therapy. Pharmacol Res 184:106452
Quintanilla RA, Tapia-Monsalves C, Vergara EH, Pérez MJ, Aranguiz A (2020) Truncated tau induces mitochondrial transport failure through the impairment of TRAK2 protein and bioenergetics decline in neuronal cells. Front Cell Neurosci 14:175. https://doi.org/10.3389/fncel.2020.00175
Hirokawa N, Niwa S, Tanaka Y (2010) Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68(4):610–638. https://doi.org/10.1016/j.neuron.2010.09.039
Vale RD, Reese TS, Sheetz MP (1985) Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42(1):39–50. https://doi.org/10.1016/s0092-8674(85)80099-4
Oeding SJ, Majstrowicz K, Hu XP, Schwarz V, Freitag A, Honnert U, Nikolaus P, Bähler M (2018) Identification of Miro1 and Miro2 as mitochondrial receptors for myosin XIX. J Cell Sci 131(17):jcs219469. https://doi.org/10.1242/jcs.219469
Mou Y, Dein J, Chen Z, Jagdale M, Li XJ (2021) MFN2 deficiency impairs mitochondrial transport and downregulates motor protein expression in human spinal motor neurons. Front Mol Neurosci 14:727552. https://doi.org/10.3389/fnmol.2021.727552
Pekkurnaz G, Wang X (2022) Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat Metab 4(7):802–812. https://doi.org/10.1038/s42255-022-00594-w
Vallee RB, McKenney RJ, Ori-McKenney KM (2012) Multiple modes of cytoplasmic dynein regulation. Nat Cell Biol 14(3):224–230. https://doi.org/10.1038/ncb2420
Canty JT, Yildiz A (2020) Activation and regulation of cytoplasmic dynein. Trends Biochem Sci 45(5):440–453. https://doi.org/10.1016/j.tibs.2020.02.002
Cason SE, Holzbaur ELF (2022) Selective motor activation in organelle transport along axons. Nat Rev Mol Cell Biol 23(11):699–714. https://doi.org/10.1038/s41580-022-00491-w
Kumar Sharma R, Chafik A, Bertolin G (2022) Mitochondrial transport, partitioning, and quality control at the heart of cell proliferation and fate acquisition. Am J Physiol Cell Physiol 322(2):C311–C325. https://doi.org/10.1152/ajpcell.00256.2021
López-Doménech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT (2018) Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J 37(3):321–336. https://doi.org/10.15252/embj.201696380
Loss O, Stephenson FA (2017) Developmental changes in trak-mediated mitochondrial transport in neurons. Mol Cell Neurosci 80:134–147. https://doi.org/10.1016/j.mcn.2017.03.006
Franker MA, Hoogenraad CC (2013) Microtubule-based transport - basic mechanisms, traffic rules and role in neurological pathogenesis. J Cell Sci 126(Pt 11):2319–2329. https://doi.org/10.1242/jcs.115030
Sheng ZH (2017) The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol 27(6):403–416. https://doi.org/10.1016/j.tcb.2017.01.005
Kruppa AJ, Buss F (2021) Motor proteins at the mitochondria-cytoskeleton interface. J Cell Sci 134(7):jcs226084. https://doi.org/10.1242/jcs.226084
Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J et al (2014) Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A 111(35):E3631-3640. https://doi.org/10.1073/pnas.1402449111
Canty JT, Hensley A, Aslan M, Jack A, Yildiz A (2023) TRAK adaptors regulate the recruitment and activation of dynein and kinesin in mitochondrial transport. Nat Commun 14(1):1376
Smith GM, Gallo G (2018) The role of mitochondria in axon development and regeneration. Dev Neurobiol 78(3):221–237. https://doi.org/10.1002/dneu.22546
Verreet T, Weaver CJ, Hino H, Hibi M, Poulain FE (2019) Syntaphilin-mediated docking of mitochondria at the growth cone is dispensable for axon elongation in vivo. eNeuro 6(5):ENEURO.0026-19.2019
Sainath R, Armijo-Weingart L, Ketscheck A, Xu Z, Li S, Gallo G (2017) Chondroitin sulfate proteoglycans negatively regulate the positioning of mitochondria and endoplasmic reticulum to distal axons. Dev Neurobiol 77(12):1351–1370
Li Q, Gao S (2017) Mitochondrial Dysfunction in Ischemic Stroke. In: Lapchak PA, Yang G-Y (eds) Translational research in stroke. Springer Singapore, Singapore, pp 201–221
Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39(11):3057–3063. https://doi.org/10.1161/STROKEAHA.108.520114
Rugarli EI, Langer T (2012) Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31(6):1336–1349. https://doi.org/10.1038/emboj.2012.38
Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY (2018) Autophagy in ischemic stroke. Prog Neurobiol 163–164:98–117. https://doi.org/10.1016/j.pneurobio.2018.01.001
Lin MY, Cheng XT, Tammineni P, Xie Y, Zhou B, Cai Q, Sheng ZH (2017) Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 94(3):595-610.e6. https://doi.org/10.1016/j.neuron.2017.04.004
Han SM, Baig HS, Hammarlund M (2016) Mitochondria localize to injured axons to support regeneration. Neuron 92(6):1308–1323. https://doi.org/10.1016/j.neuron.2016.11.025
Guedes-Dias P, Holzbaur ELF (2019) Axonal transport: driving synaptic function. Science 366(6462):eaaw9997. https://doi.org/10.1126/science.aaw9997
Chen M, Li Y, Yang M, Chen X, Chen Y, Yang F, Lu S, Yao S et al (2016) A new method for quantifying mitochondrial axonal transport. Protein Cell 7(11):804–819. https://doi.org/10.1007/s13238-016-0268-3
Zheng YR, Zhang XN, Chen Z (2019) Mitochondrial transport serves as a mitochondrial quality control strategy in axons: implications for central nervous system disorders. CNS Neurosci Ther 25(7):876–886. https://doi.org/10.1111/cns.13122
Chien L, Liang MZ, Chang CY, Wang C, Chen L (2018) Mitochondrial therapy promotes regeneration of injured hippocampal neurons. Biochim Biophys Acta Mol Basis Dis 1864(9 Pt B):3001–3012
Shi X, Zhao M, Fu C, Fu A (2017) Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 34:91–100
Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, Rabchevsky AG (2018) Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma 35(15):1800–1818
Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, Kuo SJ, Chen ST et al (2019) Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res 38(1):30
Sun C, Liu X, Wang B, Wang Z, Liu Y, Di C, Si J, Li H et al (2019) Endocytosis-mediated mitochondrial transplantation: transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 9(12):3595–3607
Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, Wang Q (2019) Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res 356:322–331
Bi Y, Guo X, Zhang M, Zhu K, Shi C, Fan B, Wu Y, Yang Z et al (2021) Bone marrow derived-mesenchymal stem cell improves diabetes-associated fatty liver via mitochondria transformation in mice. Stem Cell Res Ther 12(1):602
Robicsek O, Ene HM, Karry R, Ytzhaki O, Asor E, McPhie D, Cohen BM, Ben-Yehuda R et al (2018) Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues deficits in a rat model of the disorder. Schizophr Bull 44(2):432–442
Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK et al (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21(20):7533. https://doi.org/10.3390/ijms21207533
Fischer T, Stern C, Freund P, Schubert M, Sutter R (2021) Wallerian degeneration in cervical spinal cord tracts is commonly seen in routine T2-weighted MRI after traumatic spinal cord injury and is associated with impairment in a retrospective study. Eur Radiol 31(5):2923–2932. https://doi.org/10.1007/s00330-020-07388-2
Zhang K, Jiang M, Fang Y (2021) The drama of Wallerian degeneration: the cast, crew, and script. Annu Rev Genet 55:93–113. https://doi.org/10.1146/annurev-genet-071819-103917
Koliatsos VE, Alexandris AS (2019) Wallerian degeneration as a therapeutic target in traumatic brain injury. Curr Opin Neurol 32(6):786–795. https://doi.org/10.1097/WCO.0000000000000763
Bradshaw DV Jr, Knutsen AK, Korotcov A, Sullivan GM, Radomski KL, Dardzinski BJ, Zi X, McDaniel DP et al (2021) Genetic inactivation of SARM1 axon degeneration pathway improves outcome trajectory after experimental traumatic brain injury based on pathological, radiological, and functional measures. Acta Neuropathol Commun 9(1):89. https://doi.org/10.1186/s40478-021-01193-8
Merlini E, Coleman MP, Loreto A (2022) Mitochondrial dysfunction as a trigger of programmed axon death. Trends Neurosci 45(1):53–63. https://doi.org/10.1016/j.tins.2021.10.014
Campbell G, Mahad DJ (2018) Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis. FEBS Lett 592(7):1113–1121. https://doi.org/10.1002/1873-3468.13013
Geden MJ, Deshmukh M (2016) Axon degeneration: context defines distinct pathways. Curr Opin Neurobiol 39:108–115. https://doi.org/10.1016/j.conb.2016.05.002
Wang B, Huang M, Shang D, Yan X, Zhao B, Zhang X (2021) Mitochondrial behavior in axon degeneration and regeneration. Front Aging Neurosci 13:650038. https://doi.org/10.3389/fnagi.2021.650038
Rawson RL, Yam L, Weimer RM, Bend EG, Hartwieg E, Horvitz HR, Clark SG, Jorgensen EM (2014) Axons degenerate in the absence of mitochondria in C. elegans. Curr Biol 24(7):760–5. https://doi.org/10.1016/j.cub.2014.02.025
Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR (2012) WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol 22(7):596–600. https://doi.org/10.1016/j.cub.2012.02.043
Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL et al (2016) The mammalian-specific protein Armcx1 regulates mitochondrial transport during axon regeneration. Neuron 92(6):1294–1307. https://doi.org/10.1016/j.neuron.2016.10.060
Kalinski AL, Kar AN, Craver J, Tosolini AP, Sleigh JN, Lee SJ, Hawthorne A, Brito-Vargas P et al (2019) Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol 218(6):1871–1890. https://doi.org/10.1083/jcb.201702187
Krauss R, Bosanac T, Devraj R, Engber T, Hughes RO (2020) Axons Matter: The promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol Sci 41(4):281–293. https://doi.org/10.1016/j.tips.2020.01.006
O’Donnell KC, Vargas ME, Sagasti A (2013) WldS and PGC-1α regulate mitochondrial transport and oxidation state after axonal injury. J Neurosci 33(37):14778–14790. https://doi.org/10.1523/JNEUROSCI.1331-13.2013
Cavallucci V, Bisicchia E, Cencioni MT, Ferri A, Latini L, Nobili A, Biamonte F, Nazio F et al (2014) Acute focal brain damage alters mitochondrial dynamics and autophagy in axotomized neurons. Cell Death Dis 5(11):e1545. https://doi.org/10.1038/cddis.2014.511
Slater PG, Domínguez-Romero ME, Villarreal M, Eisner V, Larraín J (2022) Mitochondrial function in spinal cord injury and regeneration. Cell Mol Life Sci 79(5):239. https://doi.org/10.1007/s00018-022-04261-x
Das S, Boczan J, Gerwin C, Zald PB, Sheng ZH (2003) Regional and developmental regulation of syntaphilin expression in the brain: a candidate molecular element of synaptic functional differentiation. Brain Res Mol Brain Res 116(1–2):38–49. https://doi.org/10.1016/s0169-328x(03)00212-2
Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH (2013) Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep 4(3):413–419. https://doi.org/10.1016/j.celrep.2013.06.040
Li YK, Zou J, Ye DM, Zeng Y, Chen CY, Luo GF, Zeng X (2020) Human p21-activated kinase 5 (PAK5) expression and potential mechanisms in relevant cancers: basic and clinical perspectives for molecular cancer therapeutics. Life Sci 241:117113. https://doi.org/10.1016/j.lfs.2019.117113
Li D, Pan Y, Huang Y, Zhang P, Fang X (2018) PAK5 induces EMT and promotes cell migration and invasion by activating the PI3K/AKT pathway in ovarian cancer. Anal Cell Pathol (Amst) 2018:8073124. https://doi.org/10.1155/2018/8073124
Han K, Zhou Y, Tseng KF, Hu H, Li K, Wang Y, Gan Z, Lin S et al (2018) PAK5 overexpression is associated with lung metastasis in osteosarcoma. Oncol Lett 15(2):2202–2210. https://doi.org/10.3892/ol.2017.7545
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169(3):381–405. https://doi.org/10.1016/j.cell.2017.04.001
Zhao Y, Wang Q, Xie C, Cai Y, Chen X, Hou Y, He L, Li J et al (2021) Peptide ligands targeting FGF receptors promote recovery from dorsal root crush injury via AKT/mTOR signaling. Theranostics 11(20):10125–10147. https://doi.org/10.7150/thno.62525
Yang L, Lei JF, Ouyang JY, Li MZ, Zhan Y, Feng XF, Lu Y, Li MC et al (2021) Effect of neurorepair for motor functional recovery enhanced by total saponins from Trillium tschonoskii maxim. Treatment in a rat model of focal ischemia. Front Pharmacol 12:763181. https://doi.org/10.3389/fphar.2021.763181
Yao Z, Yuan W, Xu J, Tong W, Mi J, Ho PC, Chow DHK, Li Y et al (2022) Magnesium-encapsulated injectable hydrogel and 3D-engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv Sci (Weinh) 9(21):e2202102. https://doi.org/10.1002/advs.202202102
Chen S, Owens GC, Crossin KL, Edelman DB (2007) Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci 36(4):472–483. https://doi.org/10.1016/j.mcn.2007.08.004
Chen S, Owens GC, Edelman DB (2008) Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS One 3(7):e2804. https://doi.org/10.1371/journal.pone.0002804
Acknowledgements
We thank Shaoxing University for infrastructure support.
Funding
Zhejiang Provincial Natural Science Foundation of China supported this study under grant no. LY19H170001.
Author information
Authors and Affiliations
Contributions
All authors agreed to publish this article. XL designed the study. QL, YZ, BOAB, MH, and XL prepared the first draft of the manuscript. All authors revised the manuscript and approved the final article.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors read and approved the final manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Q., Zhang, Y., Botchway, B.O.A. et al. Syntaphilin Inactivation Can Enhance Axonal Mitochondrial Transport to Improve Spinal Cord Injury. Mol Neurobiol 60, 6556–6565 (2023). https://doi.org/10.1007/s12035-023-03494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03494-6