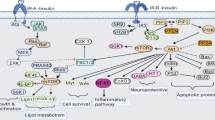
Abstract
Overexpression of PKCα has been linked to inhibit insulin signaling disrupting IRS-1 and Akt phosphorylations in skeletal muscle. PKCα inhibits IRS-1 and Akt phosphorylations, but not required for insulin-stimulated glucose transport in skeletal muscles. Inhibition of PKCα increased whereas in some studies decreased GLUT-4 levels at the plasma membrane in skeletal muscles and adipocytes. Controversial studies have reported opposite expression pattern of PKCα expression in insulin-resistant skeletal muscles. These findings indicate that the role of PKCα on insulin signaling is controversial and could be tissue specific. Evidently, studies are required to decipher the role of PKCα in regulating insulin signaling and preferably in other cellular systems. Utilizing neuronal cells, like Neuro-2a, SHSY-5Y and insulin-resistant diabetic mice brain tissues; we have demonstrated that PKCα inhibits insulin signaling, through IRS-Akt pathway in PP2A-dependent mechanism by an AS160-independent route involving 14-3-3ζ. Inhibition and silencing of PKCα improves insulin sensitivity by increasing GLUT-4 translocation to the plasma membrane and glucose uptake. PKCα regulates GSK3 isoforms in an opposite manner in insulin-sensitive and in insulin-resistant condition. Higher activity of PKCα aggravates insulin-resistant neuronal diabetic condition through GSK3β but not GSK3α. Our results mechanistically explored the contribution of PKCα in regulating neuronal insulin resistance and diabetes, which opens up new avenues in dealing with metabolic disorders and neurodegenerative disorders.







Similar content being viewed by others
Data Availability
My manuscript has data included as electronic supplementary material.
References
Haeusler RA, McGraw TE, Accili D (2018) Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 19:31–44. https://doi.org/10.1038/nrm.2017.89
Carnagarin R, Dharmarajan AM, Dass CR (2015) Molecular aspects of glucose homeostasis in skeletal muscle--A focus on the molecular mechanisms of insulin resistance. Mol Cell Endocrinol 417:52–62. https://doi.org/10.1016/j.mce.2015.09.004
Cipok M, Aga-Mizrachi S, Bak A et al (2006) Protein kinase Cα regulates insulin receptor signaling in skeletal muscle. Biochem Biophys Res Commun 345:817–824. https://doi.org/10.1016/J.BBRC.2006.05.008
Singh RK, Kumar S, Gautam PK et al (2017) Protein kinase C-α and the regulation of diverse cell responses. Biomol Concepts. https://doi.org/10.1515/bmc-2017-0005
Nakashima S (2002) Protein Kinase Cα (PKCα): Regulation and Biological Function. J Biochem (Tokyo) 132:669–675. https://doi.org/10.1093/oxfordjournals.jbchem.a003272
Callender JA, Newton AC (2017) Conventional protein kinase C in the brain: 40 years later. Neuronal Signal 1:NS20160005. https://doi.org/10.1042/NS20160005
Bandyopadhyay G, Standaert ML, Galloway L et al (1997) Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology 138:4721–4731. https://doi.org/10.1210/endo.138.11.5473
Goode N, Hughes K, Woodgett JR, Parker PJ (1992) Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem 267:16878–16882
Deng B, Zhu X, Zhao Y et al (2018) PKC and Rab13 mediate Ca2+ signal-regulated GLUT4 traffic. Biochem Biophys Res Commun 495:1956–1963. https://doi.org/10.1016/j.bbrc.2017.12.064
Nishizaki T (2018) Cellular Physiology and Biochemistry Cellular Physiology and Biochemistry Dioleoylphosphoethanolamine Retains Cell Surface GLUT4 by Inhibiting PKCα-Driven Internalization. Cell Physiol Biochem 46:1985–1998. https://doi.org/10.1159/000489439
Farese RV, Standaert ML, Arnold TP et al (1994) Preferential activation of microsomal diacylglycerol/protein kinase C signaling during glucose treatment (de novo phospholipid synthesis) of rat adipocytes. J Clin Investig 93:1894–1899. https://doi.org/10.1172/JCI117180
Chen XW, Leto D, Xiao J et al (2011) Exocyst function regulated by effector phosphorylation. Nat Cell Biol 13:580–588. https://doi.org/10.1038/ncb2226
Avignon A, Yamada K, Zhou X et al (1996) Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes 45:1396–1404. https://doi.org/10.2337/diab.45.10.1396
Cooper DR, Watson JE, Dao ML (1993) Decreased expression of protein kinase-C alpha, beta, and epsilon in soleus muscle of Zucker obese (fa/fa) rats. Endocrinology 133:2241–2247. https://doi.org/10.1210/endo.133.5.8404676
Gupta A, Bisht B, Dey CS (2011) Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology 60:910–920. https://doi.org/10.1016/J.NEUROPHARM.2011.01.033
Gupta A, Dey CS (2012) PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell 23:3882–3898. https://doi.org/10.1091/mbc.E12-05-0337
Gupta A, Bisht B, Dey CS (2012) Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim Biophys Acta (BBA) - Mol Basis Dis 1822:1030–1037. https://doi.org/10.1016/j.bbadis.2012.02.011
Heni M, Kullmann S, Preissl H et al (2015) Impaired insulin action in the human brain: Causes and metabolic consequences. Nat Rev Endocrinol 11:701–711. https://doi.org/10.1038/nrendo.2015.173
Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab TEM 16:59–65. https://doi.org/10.1016/j.tem.2005.01.008
Mishra D, Dey CS (2019) Protein Kinase C Attenuates Insulin Signalling Cascade in Insulin-Sensitive and Insulin-Resistant Neuro-2a Cells. J Mol Neurosci 69:470–477. https://doi.org/10.1007/s12031-019-01377-x
Luo B, Prescott SM, Topham MK (2003) Association of diacylglycerol kinase ζ with protein kinase C α. J Cell Biol 160:929–937. https://doi.org/10.1083/jcb.200208120
Cleland PJ, Abel KC, Rattigan S, Clark MG (1990) Long-term treatment of isolated rat soleus muscle with phorbol ester leads to loss of contraction-induced glucose transport. Biochem J 267:659–663. https://doi.org/10.1042/bj2670659
Klip A, Sun Y, Chiu TT, Foley KP (2014) Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol 306:C879-886. https://doi.org/10.1152/ajpcell.00069.2014
Watson RT, Kanzaki M, Pessin JE (2004) Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev 25:177–204. https://doi.org/10.1210/er.2003-0011
Lim GE, Piske M, Lulo JE et al (2016) Ywhaz/14-3-3ζ deletion improves glucose tolerance through a GLP-1-dependent mechanism. Endocrinology 157:2649–2659. https://doi.org/10.1210/en.2016-1016
Gannon-Murakami L, Murakami K (2002) Selective association of protein kinase C with 14-3-3 ζ in neuronally differentiated PC12 cells: Stimulatory and inhibitory effect of 14-3-3 ζ in vivo. J BiolChem 277(26):23116–23122. https://doi.org/10.1074/jbc.M201478200
Manglani K, Dey CS (2020) Tankyrase inhibition augments neuronal insulin sensitivity and glucose uptake via AMPK-AS160 mediated pathway. Neurochem Int 141:104854. https://doi.org/10.1016/j.neuint.2020.104854
Chin JE, Liu F, Roth RA (1994) Activation of protein kinase C alpha inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol Endocrinol Baltim Md 8:51–58. https://doi.org/10.1210/mend.8.1.7512195
Zheng W-H, Kar S, Quirion R (2000) Stimulation of Protein Kinase C Modulates Insulin-like Growth Factor-1-induced Akt Activation in PC12 Cells*. J Biol Chem 275:13377–13385. https://doi.org/10.1074/jbc.275.18.13377
Rosenzweig T, Braiman L, Bak A et al (2002) Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes 51:1921–1930. https://doi.org/10.2337/diabetes.51.6.1921
Hsu AH, Lum MA, Shim K-S et al (2018) Crosstalk between PKCα and PI3K/AKT signaling is tumor suppressive in the endometrium. Cell Rep 24:655–669. https://doi.org/10.1016/j.celrep.2018.06.067
Liu R, Zhou XW, Tanila H et al (2008) Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology: In Focus. J Cell Mol Med 12:241–257. https://doi.org/10.1111/j.1582-4934.2008.00249.x
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5:1–8. https://doi.org/10.1186/gb-2004-5-3-214
Sirover MA (2012) Subcellular dynamics of multifunctional protein regulation: Mechanisms of GAPDH intracellular translocation. J Cell Biochem 113:2193–2200. https://doi.org/10.1002/jcb.24113
Tan SX, Ng Y, Meoli CC et al (2012) Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J Biol Chem 287:6128–6138. https://doi.org/10.1074/jbc.M111.318238
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274. https://doi.org/10.1016/j.cell.2007.06.009
Hu X, Hipolito S, Lynn R et al (2004) Relative gene-silencing efficiencies of small interfering RNAs targeting sense and antisense transcripts from the same genetic locus. Nucleic Acids Res 32:4609–4617. https://doi.org/10.1093/nar/gkh790
Sperbera BR, Leight S, Goedert M, Lee VMY (1995) Glycogen synthase kinase-3β phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett 197:149–153. https://doi.org/10.1016/0304-3940(95)11902-9
Hernandez F, Lucas JJ, Avila J (2013) GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis JAD 33 Suppl 1:S141–144. https://doi.org/10.3233/JAD-2012-129025
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104:1433–1439. https://doi.org/10.1111/j.1471-4159.2007.05194.x
Brion JP (1998) Neurofibrillary tangles and Alzheimer’s disease. Eur Neurol 40:130–140. https://doi.org/10.1159/000007969
Young LH, Balin BJ, Weis MT (2005) Gö 6983: a fast acting protein kinase C inhibitor that attenuates myocardial ischemia/reperfusion injury. Cardiovasc Drug Rev 23:255–272. https://doi.org/10.1111/j.1527-3466.2005.tb00170.x
Bessa C, Soares J, Raimundo L et al (2018) Discovery of a small-molecule protein kinase Cδ-selective activator with promising application in colon cancer therapy. Cell Death Dis 9:1–16. https://doi.org/10.1038/s41419-017-0154-9
Cabedo H, Miñana M-D, Grau E et al (1996) Protein kinase C isoforms and cell proliferation in neuroblastoma cells. Mol Brain Res 37:125–133. https://doi.org/10.1016/0169-328X(95)00290-9
Schmitz-Peiffer C (2020) Deconstructing the Role of PKC Epsilon in Glucose Homeostasis. Trends Endocrinol Metab:1–13. https://doi.org/10.1016/j.tem.2020.01.016
ter Horst KW, Gilijamse PW, Versteeg RI et al (2017) Hepatic Diacylglycerol-Associated Protein Kinase Cε Translocation Links Hepatic Steatosis to Hepatic Insulin Resistance in Humans. Cell Rep 19:1997–2004. https://doi.org/10.1016/j.celrep.2017.05.035
Salcedo-Tello P, Ortiz-Matamoros A, Arias C (2011) GSK3 Function in the Brain during Development, Neuronal Plasticity, and Neurodegeneration. Int J Alzheimers Dis 2011. https://doi.org/10.4061/2011/189728
Ma YX, Wang XL, Chen JQ et al (2017) Differential roles of glycogen synthase kinase 3 subtypes alpha and beta in cortical development. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00391
Maurer U, Preiss F, Brauns-Schubert P et al (2014) GSK-3 -at the crossroads of cell death and survival. J Cell Sci 127:1369–1378. https://doi.org/10.1242/jcs.138057
Callender JA, Yang Y, Lordén G et al Protein kinase Cα gain-of-function variant in Alzheimer’s disease displays enhanced catalysis by a mechanism that evades down-regulation. National Acad Sciences. https://doi.org/10.1073/pnas.1805046115
Alfonso SI, Callender JA, Hooli B et al (2016) Gain-of-function mutations in protein kinase Cα (PKCα) may promote synaptic defects in Alzheimer’s disease. Sci Signal 9:ra47. https://doi.org/10.1126/scisignal.aaf6209
Su R, Han ZY, Fan JP, Zhang YL (2010) A possible role of myristoylated alanine-rich C kinase substrate in endocytic pathway of Alzheimer’s disease. Neurosci Bull 26:338–344. https://doi.org/10.1007/s12264-010-0131-0
Chin JE, Liu F, Roth RA (1994) Activation of protein kinase Cα inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol Endocrinol 8:51–58. https://doi.org/10.1210/mend.8.1.7512195
Gassaway BM, Petersen MC, Surovtseva YV et al PKCe contributes to lipid-induced insulin resistance through cross talk with p70S6K and through previously unknown regulators of insulin signaling. Proc Natl Acad Sci 115(38):E8996–E9005. https://doi.org/10.1073/pnas.1804379115
Sheetz MJ, Aiello LP, Davis MD et al (2013) The effect of the Oral PKC β inhibitor ruboxistaurin on vision loss in two phase 3 studies. Investig Ophthalmol Vis Sci 54:1750–1757. https://doi.org/10.1167/iovs.12-11055
Steen E, Terry BM, Rivera EJ et al (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - Is this type 3 diabetes? J Alzheimers Dis 7:63–80. https://doi.org/10.3233/JAD-2005-7107
Varshney P, Dey CS (2016) P21-activated kinase 2 (PAK2) regulates glucose uptake and insulin sensitivity in neuronal cells. Mol Cell Endocrinol 429:50–61. https://doi.org/10.1016/j.mce.2016.03.035
Arora A, Dey CS (2016) SIRT2 regulates insulin sensitivity in insulin resistant neuronal cells. Biochem Biophys Res Commun 474:747–752. https://doi.org/10.1016/j.bbrc.2016.05.029
Zhou H, Wang Y, Zhou Q et al (2016) Down-Regulation of Protein Kinase C-ε by Prolonged Incubation with PMA Inhibits the Proliferation of Vascular Smooth Muscle Cells. Cell Physiol Biochem 40:379–390. https://doi.org/10.1159/000452553
Silva G, Ferraresi C, de Almeida RT et al (2020) Insulin resistance is improved in high-fat fed mice by photobiomodulation therapy at 630 nm. J Biophotonics 13:1–16. https://doi.org/10.1002/jbio.201960140
Mujawdiya PK, Sharma P, Sharad S, Kapur S (2020) Reversal of increase in intestinal permeability by mangifera indica seed kernel extract in high-fat diet-induced obese mice. Pharmaceuticals 13:1–20. https://doi.org/10.3390/ph13080190
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Sharma M, Dey CS (2021) Role of Akt isoforms in neuronal insulin signaling and resistance. Cell Mol Life Sci 78:7873–7898. https://doi.org/10.1007/s00018-021-03993-6
Irrcher I, Adhihetty PJ, Joseph A-M et al (2003) Regulation of Mitochondrial Biogenesis in Muscle by Endurance Exercise. Sports Med 33:783–793. https://doi.org/10.2165/00007256-200333110-00001
Goel HL, Dey CS (2002) Insulin stimulates spreading of skeletal muscle cells involving the activation of focal adhesion kinase, phosphatidylinositol 3-kinase and extracellular signal regulated kinases. J Cell Physiol 193:187–198. https://doi.org/10.1002/jcp.10161
Acknowledgements
We are grateful to Indian Institute of Technology-Delhi for its support. We would like to thank Dr.Prosenjit Mondal, Indian Institute of Technology-Mandi, Himachal Pradesh, India, for providing us with normal diet (ND) and high fat fed diet (HFD) mouse brain samples. We are grateful to Central Instrumentation Facility (CIF), University of Delhi, South Campus, New Delhi, India, for Confocal Microscopy. We would like to thank Dr. Chinmoy Mukhopadhyay, Jawaharalal Nehru University, New Delhi, for providing us with Caveolin-1 antibody.
Funding
D.M. was a recipient of Senior Research Fellowship from CSIR, Government of India. I.R. is a recipient of Senior Research Fellowship from DBT, Government of India. C.S.D is supported by a grant from Indian Council of Medical Research, Government of India, New Delhi, 5/4/5-17/Diab./20-NCD-III.
Author information
Authors and Affiliations
Contributions
C.S.D. conceived the idea, D.M. did all the experiments; I.R. aided in interpreting the results. C.S.D., D.M. and I.R. wrote the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were performed following the guidelines prescribed by CPCSEA with the approval of the Internal Animal Ethics Committee, Visva-Bharati (IAEC/VB/2017/01).
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
Competing Interest
Authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, D., Reddy, I. & Dey, C.S. PKCα Isoform Inhibits Insulin Signaling and Aggravates Neuronal Insulin Resistance. Mol Neurobiol 60, 6642–6659 (2023). https://doi.org/10.1007/s12035-023-03486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03486-6