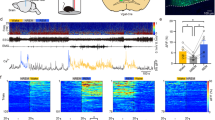
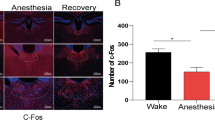
Abstract
The mechanism underlying the hypnosis effect of propofol is still not fully understood. In essence, the nucleus accumbens (NAc) is crucial for regulating wakefulness and may be directly engaged in the principle of general anesthesia. However, the role of NAc in the process of propofol-induced anesthesia is still unknown. We used immunofluorescence, western blotting, and patch-clamp to access the activities of NAc GABAergic neurons during propofol anesthesia, and then we utilized chemogenetic and optogenetic methods to explore the role of NAc GABAergic neurons in regulating propofol-induced general anesthesia states. Moreover, we also conducted behavioral tests to analyze anesthetic induction and emergence. We found out that c-Fos expression was considerably dropped in NAc GABAergic neurons after propofol injection. Meanwhile, patch-clamp recording of brain slices showed that firing frequency induced by step currents in NAc GABAergic neurons significantly decreased after propofol perfusion. Notably, chemically selective stimulation of NAc GABAergic neurons during propofol anesthesia lowered propofol sensitivity, prolonged the induction of propofol anesthesia, and facilitated recovery; the inhibition of NAc GABAergic neurons exerted opposite effects. Furthermore, optogenetic activation of NAc GABAergic neurons promoted emergence whereas the result of optogenetic inhibition was the opposite. Our results demonstrate that NAc GABAergic neurons modulate propofol anesthesia induction and emergence.






Similar content being viewed by others
Data Availability
The datasets and/or analysis are available from the corresponding author upon reasonable request.
References
Bao WW, Jiang S, Qu WM, Li WX, Miao CH, Huang ZA-O (2023) Understanding the neural mechanisms of general anesthesia from interaction with sleep-wake state: a decade of discovery. Pharmacol Rev 75(3):532–553 (1521-0081 (Electronic)). https://doi.org/10.1124/pharmrev.122.000717
Brown EN, Purdon PL, Van Dort CJ (2011) General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 34:601–628. https://doi.org/10.1146/annurev-neuro-060909-153200
Yang QZ, Zhou F, Li A, Dong HL (2022) Neural substrates for the regulation of sleep and general anesthesia. Curr Neuropharmacol 20(1):72–84. https://doi.org/10.2174/1570159x19666211214144639
Berthoud MC, Reilly CS (1992) Adverse effects of general anesthetics. Drug Saf 7(6):434–459. https://doi.org/10.2165/00002018-199207060-00005
Sahinovic MM, Struys M, Absalom AR (2018) Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet 57(12):1539–1558. https://doi.org/10.1007/s40262-018-0672-3
Moody OA, Zhang ER, Vincent KF, Kato R, Melonakos ED, Nehs CJ, Solt K (2021) The neural circuits underlying general anesthesia and sleep. Anesth Analg 132(5):1254–1264. https://doi.org/10.1213/ANE.0000000000005361
Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9(5):370–386. https://doi.org/10.1038/nrn2372
Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M et al (2009) The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci 29(7):2177–2187. https://doi.org/10.1523/JNEUROSCI.4997-08.2009
Liu Y, Chen B, Cai Y, Han Y, Xia Y, Li N, Fan B, Yuan T et al (2021) Activation of anterior thalamic reticular nucleus GABAergic neurons promotes arousal from propofol anesthesia in mice. Acta Biochim Biophys Sin 53(7):883–892. https://doi.org/10.1093/abbs/gmab056
Flores FJ, Hartnack KE, Fath AB, Kim S-E, Wilson MA, Brown EN, Purdon PL (2017) Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A 114(32):E6660–E6668. https://doi.org/10.1073/pnas.1700148114
Wang L, Zhang W, Wu Y, Gao Y, Sun N, Ding H, Ren J, Yu L et al (2021) Cholinergic-induced specific oscillations in the medial prefrontal cortex to reverse propofol anesthesia. Front Neurosci 15:664410. https://doi.org/10.3389/fnins.2021.664410
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016) The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 68(3):816–871. https://doi.org/10.1124/pr.116.012484
David Smith A, Paul Bolam J (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13(7):259–265. https://doi.org/10.1016/0166-2236(90)90106-K
Floresco SB (2015) The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66:25–52. https://doi.org/10.1146/annurev-psych-010213-115159
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA et al (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475(7356):377–380. https://doi.org/10.1038/nature10194
McCullough KM, Missig G, Robble MA, Foilb AR, Wells AM, Hartmann J, Anderson KJ, Neve RL et al (2021) Nucleus Accumbens medium spiny neuron subtypes differentially regulate stress-associated alterations in sleep architecture. Biol Psychiatry 89(12):1138–1149. https://doi.org/10.1016/j.biopsych.2020.12.030
Seminowicz DA, Remeniuk B, Krimmel SR, Smith MT, Barrett FS, Wulff AB, Furman AJ, Geuter S et al (2019) Pain-related nucleus accumbens function: modulation by reward and sleep disruption. Pain 160(5):1196–1207. https://doi.org/10.1097/j.pain.0000000000001498
Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, Ba W, Miracca G et al (2019) GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22(1):106–119. https://doi.org/10.1038/s41593-018-0288-9
Luo YJ, Li YD, Wang L, Yang SR, Yuan XS, Wang J, Cherasse Y, Lazarus M et al (2018) Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D(1) receptors. Nat Commun 9(1):1576. https://doi.org/10.1038/s41467-018-03889-3
Yuan XS, Wang L, Dong H, Qu WM, Yang SR, Cherasse Y, Lazarus M, Schiffmann SN et al (2017) Striatal adenosine A(2A) receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. Elife 6:e29055. https://doi.org/10.7554/eLife.29055
Raguz M, Predrijevac N, Dlaka D, Oreskovic D, Rotim A, Romic D, Almahariq F, Marcinkovic P et al (2021) Structural changes in brains of patients with disorders of consciousness treated with deep brain stimulation. Sci Rep 11(1):4401. https://doi.org/10.1038/s41598-021-83873-y
Reitz SL, Wasilczuk AZ, Beh GH, Proekt A, Kelz MB (2021) Activation of preoptic tachykinin 1 neurons promotes wakefulness over sleep and volatile anesthetic-induced unconsciousness. Curr Biol 31(2):394–405 e394. https://doi.org/10.1016/j.cub.2020.10.050
Wang D, Guo Q, Zhou Y, Xu Z, Hu SW, Kong XX, Yu YM, Yang JX et al (2021) GABAergic neurons in the dorsal-intermediate lateral septum regulate sleep-wakefulness and anesthesia in mice. Anesthesiology 135(3):463–481. https://doi.org/10.1097/ALN.0000000000003868
Ma LH, Wan J, Yan J, Wang N, Liu YP, Wang HB, Zhou CH, Wu YQ (2022) Hippocampal SIRT1-mediated synaptic plasticity and glutamatergic neuronal excitability are involved in prolonged cognitive dysfunction of neonatal rats exposed to propofol. Mol Neurobiol 59(3):1938–1953. https://doi.org/10.1007/s12035-021-02684-4
Segev A, Garcia-Oscos F, Kourrich S (2016) Whole-cell patch-clamp recordings in brain slices. J Vis Exp 112:e54024. https://doi.org/10.3791/54024
Yang E, Granata D, Eckenhoff RG, Carnevale V, Covarrubias M (2018) Propofol inhibits prokaryotic voltage-gated Na(+) channels by promoting activation-coupled inactivation. J Gen Physiol 150(9):1299–1316. https://doi.org/10.1085/jgp.201711924
Kennedy D (2005) What don’t we know. Science 309:75
Hemmings HC Jr, Riegelhaupt PM, Kelz MB, Solt K, Eckenhoff RG, Orser BA, Goldstein PA (2019) Towards a comprehensive understanding of anesthetic mechanisms of action: a decade of discovery. Trends Pharmacol Sci 40(7):464–481. https://doi.org/10.1016/j.tips.2019.05.001
Bao WW, Xu W, Pan GJ, Wang TX, Han Y, Qu WM, Li WX, Huang ZL (2021) Nucleus accumbens neurons expressing dopamine D1 receptors modulate states of consciousness in sevoflurane anesthesia. Curr Biol 31(9):1893–1902 e1895. https://doi.org/10.1016/j.cub.2021.02.011
Knapska E, Radwanska K, Werka T, Kaczmarek L (2007) Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev 87(4):1113–1173. https://doi.org/10.1152/physrev.00037.2006
Bola M, Barrett AB, Pigorini A, Nobili L, Seth AK, Marchewka A (2018) Loss of consciousness is related to hyper-correlated gamma-band activity in anesthetized macaques and sleeping humans. Neuroimage 167:130–142. https://doi.org/10.1016/j.neuroimage.2017.11.030
Gray JA (1995) Dopamine release in the nucleus accumbens: the perspective from aberrations of consciousness in schizophrenia. Neuropsychologia 33(9):1143–1153. https://doi.org/10.1016/0028-3932(95)00054-7
Chen L, Li S, Zhou Y, Liu T, Cai A, Zhang Z, Xu F, Manyande A et al (2021) Neuronal mechanisms of adenosine A(2A) receptors in the loss of consciousness induced by propofol general anesthesia with functional magnetic resonance imaging. J Neurochem 156(6):1020–1032. https://doi.org/10.1111/jnc.15146
Liu X, Lauer KK, Douglas Ward B, Roberts C, Liu S, Gollapudy S, Rohloff R, Gross W et al (2017) Propofol attenuates low-frequency fluctuations of resting-state fMRI BOLD signal in the anterior frontal cortex upon loss of consciousness. Neuroimage 147:295–301. https://doi.org/10.1016/j.neuroimage.2016.12.043
Leung LS, Luo T, Ma J, Herrick I (2014) Brain areas that influence general anesthesia. Prog Neurobiol 122:24–44. https://doi.org/10.1016/j.pneurobio.2014.08.001
Engelhardt T, Lowe PR, Galley HF, Webster NR (2006) Inhibition of neuronal nitric oxide synthase reduces the propofol requirements in wild-type and nNOS knockout mice. Eur J Anaesthesiol 23(3):197–201. https://doi.org/10.1017/S0265021505002188
Koukouli F, Rooy M, Changeux J-P, Maskos U (2016) Nicotinic receptors in mouse prefrontal cortex modulate ultraslow fluctuations related to conscious processing. Proc Natl Acad Sci 113(51):14823–14828. https://doi.org/10.1073/pnas.1614417113
Laverty D, Desai R, Uchanski T, Masiulis S, Stec WJ, Malinauskas T, Zivanov J, Pardon E et al (2019) Cryo-EM structure of the human alpha1beta3gamma2 GABA(A) receptor in a lipid bilayer. Nature 565(7740):516–520. https://doi.org/10.1038/s41586-018-0833-4
Pavel MA, Petersen EN, Wang H, Lerner RA, Hansen SB (2020) Studies on the mechanism of general anesthesia. Proc Natl Acad Sci U S A 117(24):13757–13766. https://doi.org/10.1073/pnas.2004259117
Stock L, Hosoume J, Cirqueira L, Treptow W (2018) Binding of the general anesthetic sevoflurane to ion channels. PLoS Comput Biol 14(11):e1006605. https://doi.org/10.1371/journal.pcbi.1006605
Jiang-Xie LF, Yin L, Zhao S, Prevosto V, Han BX, Dzirasa K, Wang F (2019) A common neuroendocrine substrate for diverse general anesthetics and sleep. Neuron 102(5):1053–1065 e1054. https://doi.org/10.1016/j.neuron.2019.03.033
Hammes J, Theis H, Giehl K, Hoenig MC, Greuel A, Tittgemeyer M, Timmermann L, Fink GR et al (2019) Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain 142(3):733–743. https://doi.org/10.1093/brain/awz007
Brand PA, Paris A, Bein B, Meybohm P, Scholz J, Ohnesorge H, Tonner PH (2008) Propofol sedation is reduced by delta9-tetrahydrocannabinol in mice. Anesth Analg 107(1):102–106. https://doi.org/10.1213/ane.0b013e318173287a
Mansouri MT, Fidler JA, Meng QC, Eckenhoff RG, Garcia PS (2019) Sex effects on behavioral markers of emergence from propofol and isoflurane anesthesia in rats. Behav Brain Res 367:59–67. https://doi.org/10.1016/j.bbr.2019.03.029
Acknowledgements
We thank Jia-Tao Lin, Shuai Li, and Cheng-Hua Zhou for their assistance during the preparation of this manuscript.
Funding
This research was supported by the National College Students Innovation and Entrepreneurship Training Program (202110313012Z) and the National Natural Science Foundation of China (81971051, 82171191, 82101281, 82271233, 81970994).
Author information
Authors and Affiliations
Contributions
Under the guidance and supervision of Yu-Qing Wu, Hui Zheng, and Shuai Li, Jing Yan completed its design, coordination, and optogenetic/chemical genetics experiment. Bei-Ning Hang and Lin-Hui Ma performed the propofol anesthesia, sample preparation, immunofluorescence staining, and electroencephalogram analysis. Jia-Tao Lin performed in vitro electrophysiology recordings. All the authors participated in data collection and statistical analysis. The manuscript was written by Jing Yan. All the authors have read and approved the final manuscript. Yu-Qing Wu, Hui Zheng, and Shuai Li secured funding for the project.
Corresponding authors
Ethics declarations
Ethics Approval
Animal studies were authorized by the Institutional Animal Care and Use Committee of Xuzhou Medical University (IACUC no. 202205A249).
Consent to Participate
Not applicable, as no individual participant’s data is presented.
Consent for Publication
Not applicable, as no individual participant’s data is presented.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, J., Hang, BN., Ma, LH. et al. GABAergic Neurons in the Nucleus Accumbens are Involved in the General Anesthesia Effect of Propofol. Mol Neurobiol 60, 5789–5804 (2023). https://doi.org/10.1007/s12035-023-03445-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03445-1