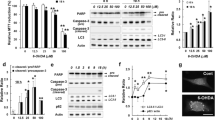
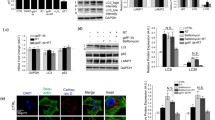
Abstract
Defective autophagy relates to the pathogenesis of Parkinson’s disease (PD), a typical neurodegenerative disease. Our recent study has demonstrated that PD toxins (6-OHDA, MPP+, or rotenone) induce neuronal apoptosis by impeding the AMPK/Akt-mTOR signaling. Here, we show that treatment with 6-OHDA, MPP+, or rotenone triggered decreases of ATG5/LC3-II and autophagosome formation with a concomitant increase of p62 in PC12, SH-SY5Y cells, and primary neurons, suggesting inhibition of autophagy. Interestingly, overexpression of wild-type ATG5 attenuated the inhibitory effect of PD toxins on autophagy, reducing neuronal apoptosis. The effects of PD toxins on autophagy and apoptosis were found to be associated with activation of PTEN and inactivation of Akt. Overexpression of dominant negative PTEN, constitutively active Akt and/or pretreatment with rapamycin rescued the cells from PD toxins-induced downregulation of ATG5/LC3-II and upregulation of p62, as well as consequential autophagosome diminishment and apoptosis in the cells. The effects of PD toxins on autophagy and apoptosis linked to excessive intracellular and mitochondrial hydrogen peroxide (H2O2) production, as evidenced by using a H2O2-scavenging enzyme catalase, a mitochondrial superoxide indicator MitoSOX and a mitochondria-selective superoxide scavenger Mito-TEMPO. Furthermore, we observed that treatment with PD toxins reduced the protein level of Parkin in the cells. Knockdown of Parkin alleviated the effects of PD toxins on H2O2 production, PTEN/Akt activity, autophagy, and apoptosis in the cells, whereas overexpression of wild-type Parkin exacerbated these effects of PD toxins, implying the involvement of Parkin in the PD toxins-induced oxidative stress. Taken together, the results indicate that PD toxins can elicit mitochondrial H2O2, which can activate PTEN and inactivate Akt leading to autophagy inhibition-dependent neuronal apoptosis, and Parkin plays a critical role in this process. Our findings suggest that co-manipulation of the PTEN/Akt/autophagy signaling by antioxidants may be exploited for the prevention of neuronal loss in PD.









Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Change history
11 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12035-023-03335-6
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- AD:
-
Alzheimer disease
- AMPK:
-
AMP-activated protein kinase
- Atg:
-
Autophagy-related
- CAT:
-
Catalase
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- HA:
-
Hemagglutinin
- HD:
-
Huntington’s disease
- H2DCFDA:
-
2′7′-Dichlorodihydrofluorescein diacetate
- H2O2 :
-
Hydrogen peroxide
- IGF-1R:
-
Insulin-like growth factor-1 receptor
- IOD:
-
Integral optical density
- LC3:
-
Microtuble-associated protein 1 light chain 3
- MPP+ :
-
1-Methyl-4-phenylpyridin-1-ium
- mTOR:
-
Mammalian target of rapamycin
- PBS:
-
Phosphate-buffered saline
- PD:
-
Parkinson’s disease
- PDL:
-
Poly-D-lysine
- PI3K:
-
Phosphatidylinositol 3-kinase
- PKB/Akt:
-
Protein kinase B
- PTEN:
-
Phosphatase and tensin homologue on chromosome 10
- PINK1:
-
PTEN-induced kinase 1
- PTP1B:
-
Protein tyrosine phosphatase 1B
- ROS:
-
Reactive oxygen species
- TUNEL:
-
The terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling
References
Masaldan S, Callegari S, Dewson G (2022) Therapeutic targeting of mitophagy in Parkinson’s disease. Biochem Soc Trans 50(2):783–797. https://doi.org/10.1042/BST20211107
Zhao H, Zhao X, Liu L, Zhang H, Xuan M, Guo Z, Wang H, Liu C (2017) Neurochemical effects of the R form of α-lipoic acid and its neuroprotective mechanism in cellular models of Parkinson’s disease. Int J Biochem Cell Biol 87:86–94. https://doi.org/10.1016/j.biocel.2017.04.002
Das M, Devi KP (2021) Dihydroactinidiolide regulates Nrf2/HO-1 expression and inhibits caspase-3/Bax pathway to protect SH-SY5Y human neuroblastoma cells from oxidative stress induced neuronal apoptosis. Neurotoxicology 84:53–63. https://doi.org/10.1016/j.neuro.2021.02.006
Zhang K, Zhu S, Li J, Jiang T, Feng L, Pei J, Wang G, Ouyang L et al (2021) Targeting autophagy using small-molecule compounds to improve potential therapy of Parkinson’s disease. Acta Pharm Sin B 11(10):3015–3034. https://doi.org/10.1016/j.apsb.2021.02.016
Ren Q, Jiang X, Zhang S, Gao X, Paudel YN, Zhang P, Wang R, Liu K et al (2022) Neuroprotective effect of YIAEDAER peptide against Parkinson’s disease like pathology in zebrafish. Biomed Pharmacother 147:112629. https://doi.org/10.1016/j.biopha.2022.112629
Komilova NR, Angelova PR, Berezhnov AV, Stelmashchuk OA, Mirkhodjaev UZ, Houlden H, Gourine AV, Esteras N et al (2022) Metabolically induced intracellular pH changes activate mitophagy, autophagy, and cell protection in familial forms of Parkinson’s disease. FEBS J 289(3):699–711. https://doi.org/10.1111/febs.16198
Zhang H, Duan C, Yang H (2015) Defective autophagy in Parkinson’s disease: lessons from genetics. Mol Neurobiol 51(1):89–104. https://doi.org/10.1007/s12035-014-8787-5
van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, Bardien S (2017) Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson’s disease from mitochondrial dysfunction and cell death. Mol Neurobiol 54(4):2752–2762. https://doi.org/10.1007/s12035-016-9843-0
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4(11):600–609. https://doi.org/10.1038/ncpneuro0924
Corti O, Blomgren K, Poletti A, Beart PM (2020) Autophagy in neurodegeneration: new insights underpinning therapy for neurological diseases. J Neurochem 154(4):354–371. https://doi.org/10.1111/jnc.15002
Pan PY, Yue Z (2014) Genetic causes of Parkinson’s disease and their links to autophagy regulation. Parkinsonism Relat Disord 20(Suppl 1):S154-157. https://doi.org/10.1016/S1353-8020(13)70037-3
Damme M, Suntio T, Saftig P, Eskelinen EL (2015) Autophagy in neuronal cells: general principles and physiological and pathological functions. Acta Neuropathol 129(3):337–362. https://doi.org/10.1007/s00401-014-1361-4
Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24(1):24–41. https://doi.org/10.1038/cr.2013.168
Zhang Z, Miah M, Culbreth M, Aschner M (2016) Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem Res 41(1–2):409–422. https://doi.org/10.1007/s11064-016-1844-x
Suresh SN, Verma V, Sateesh S, Clement JP, Manjithaya R (2018) Neurodegenerative diseases: model organisms, pathology and autophagy. J Genet 97(3):679–701
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752. https://doi.org/10.1038/nrm2239
Panigrahi AR, Pinder SE, Chan SY, Paish EC, Robertson JF, Ellis IO (2004) The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol 204(1):93–100. https://doi.org/10.1002/path.1611
Maehama T, Dixon JE (1999) PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9(4):125–128. https://doi.org/10.1016/s0962-8924(99)01519-6
Yu X, Li S, Pang M, Du Y, Xu T, Bai T, Yang K, Hu J et al (2021) TSPAN7 exerts anti-tumor effects in bladder cancer through the PTEN/PI3K/AKT pathway. Front Oncol 10:613869. https://doi.org/10.3389/fonc.2020.613869
Bermudez Brito M, Goulielmaki E, Papakonstanti EA (2015) Focus on PTEN regulation. Front. Oncol 5:166. https://doi.org/10.3389/fonc.2015.00166
Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E (2001) The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem 276(38):35243–35246. https://doi.org/10.1074/jbc.C100319200
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, Liu J, Zhang J (2020) Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol 104(2):575–587. https://doi.org/10.1007/s00253-019-10257-8
Dong X, Zhao R, Li Y, Yu Q, Chen X, Hu X, Ma J, Chen X et al (2019) Maduramicin inactivation of Akt impairs autophagic flux leading to accumulated autophagosomes-dependent apoptosis in skeletal myoblast cells. Int J Biochem Cell Biol 114:105573. https://doi.org/10.1016/j.biocel.2019.105573
Chen Q, Yue F, Li W, Zou J, Xu T, Huang C, Zhang Y, Song K et al (2015) Potassium bisperoxo(1,10-phenanthroline)oxovanadate (bpV(phen)) induces apoptosis and pyroptosis and disrupts the p62-HDAC6 protein interaction to suppress the acetylated microtubule-dependent degradation of autophagosomes. J Biol Chem 290(43):26051–26058. https://doi.org/10.1074/jbc.M115.653568
Zhang R, Liu C, Yang L, Ji T, Zhang N, Dong X, Chen X, Ma J et al (2022) NOX2-derived hydrogen peroxide impedes the AMPK/Akt-mTOR signaling pathway contributing to cell death in neuronal cells. Cell Signal 94:110330. https://doi.org/10.1016/j.cellsig.2022.110330
Zhu Y, Hoell P, Ahlemeyer B, Sure U, Bertalanffy H, Krieglstein J (2007) Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson’s disease. Neurochem Int 50(3):507–516. https://doi.org/10.1016/j.neuint.2006.10.010
Pang D, Li C, Yang C, Zou Y, Feng B, Li L, Liu W, Geng Y et al (2019) Polyphyllin VII promotes apoptosis and autophagic cell death via ROS-inhibited AKT activity, and sensitizes glioma cells to temozolomide. Oxid Med Cell Longev 2019:1805635. https://doi.org/10.1155/2019/1805635
Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V et al (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18(5):769–782. https://doi.org/10.1038/cdd.2010.142
Ganguly U, Banerjee A, Chakrabarti SS, Kaur U, Sen O, Cappai R, Chakrabarti S (2020) Interaction of α-synuclein and Parkin in iron toxicity on SH-SY5Y cells: implications in the pathogenesis of Parkinson’s disease. Biochem J 477(6):1109–1122. https://doi.org/10.1042/BCJ20190676
Xu W, Ocak U, Gao L, Tu S, Lenahan CJ, Zhang J, Shao A (2021) Selective autophagy as a therapeutic target for neurological diseases. Cell Mol Life Sci 78(4):1369–1392. https://doi.org/10.1007/s00018-020-03667-9
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786. https://doi.org/10.1038/nature05291
Hwang CJ, Kim YE, Son DJ, Park MH, Choi DY, Park PH, Hellstrom M, Han SB et al (2017) Parkin deficiency exacerbate ethanol-induced dopaminergic neurodegeneration by P38 pathway dependent inhibition of autophagy and mitochondrial function. Redox Biol 11:456–468. https://doi.org/10.1016/j.redox.2016.12.008
Sato S, Noda S, Hattori N (2020) Pathogenic insights to Parkin-linked model mice. Neurosci Res 159:47–51. https://doi.org/10.1016/j.neures.2020.03.014
Noda S, Sato S, Fukuda T, Tada N, Uchiyama Y, Tanaka K, Hattori N (2020) Loss of Parkin contributes to mitochondrial turnover and dopaminergic neuronal loss in aged mice. Neurobiol Dis 136:104717. https://doi.org/10.1016/j.nbd.2019.104717
Chen J, Ren Y, Gui C, Zhao M, Wu X, Mao K, Li W, Zou F (2018) Phosphorylation of Parkin at serine 131 by p38 MAPK promotes mitochondrial dysfunction and neuronal death in mutant A53T alpha-synuclein model of Parkinson’s disease. Cell Death Dis 9(6):700. https://doi.org/10.1038/s41419-018-0722-7
Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T et al (2010) Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells. Lab Invest 90(5):762–773. https://doi.org/10.1038/labinvest.2010.36
Findley CM, Cudmore MJ, Ahmed A, Kontos CD (2007) VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol 27(12):2619–2626. https://doi.org/10.1161/ATVBAHA.107.150482
Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X et al (2010) Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem 285(49):38362–38373. https://doi.org/10.1074/jbc.M110.141168
Fujio Y, Walsh K (1999) Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. The J Biol Chem 274(23):16349–16354. https://doi.org/10.1074/jbc.274.23.16349
Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K (2005) Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19(4):489–501. https://doi.org/10.1101/gad.1248505
Liu L, Li F, Cardelli JA, Martin KA, Blenis J, Huang S (2006) Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 25(53):7029–7040. https://doi.org/10.1038/sj.onc.1209691
Chen L, Liu L, Luo Y, Huang S (2008) MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J Neurochem 105(1):251–261. https://doi.org/10.1111/j.1471-4159.2007.05133.x
Zhang R, Zhang N, Zhang H, Liu C, Dong X, Wang X, Zhu Y, Xu C et al (2017) Celastrol prevents cadmium-induced neuronal cell death by blocking reactive oxygen species-mediated mammalian target of rapamycin pathway. Br J Pharmacol 174(1):82–100. https://doi.org/10.1111/bph.13655
Bao L, Avshalumov MV, Rice ME (2005) Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J Neurosci 25(43):10029–10040. https://doi.org/10.1523/JNEUROSCI.2652-05.2005
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728. https://doi.org/10.1093/emboj/19.21.5720
Ma T, Li J, Xu Y, Yu C, Xu T, Wang H, Liu K, Cao N et al (2015) Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nat Cell Biol 17(11):1379–1387. https://doi.org/10.1038/ncb3256
Yin Y, Sun G, Li E, Kiselyov K, Sun D (2017) ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev 34:3–14. https://doi.org/10.1016/j.arr.2016.08.008
Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H et al (2014) Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal 26(8):1680–1689. https://doi.org/10.1016/j.cellsig.2014.04.009
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125(25):3170–3181. https://doi.org/10.1161/CIRCULATIONAHA.111.041814
Stefani A, Cerroni R, Pierantozzi M, D’Angelo V, Grandi L, Spanetta M, Galati S (2021) Deep brain stimulation in Parkinson’s disease patients and routine 6-OHDA rodent models: Synergies and pitfalls. Eur J Neurosci 53(7):2322–2343. https://doi.org/10.1111/ejn.14950
Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, Wang H, Gong X et al (2016) MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson’s disease by targeting to Bim. Brain Pathol 26(2):167–176. https://doi.org/10.1111/bpa.12267
Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA et al (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79. https://doi.org/10.1016/j.neuron.2009.12.023
Azam S, Haque ME, Cho DY, Kim JS, Jakaria M, Kim IS, Choi DK (2022) Dioscin-mediated autophagy alleviates MPP+-induced neuronal degeneration: an in vitro Parkinson’s disease model. Molecules 27(9):2827. https://doi.org/10.3390/molecules27092827
Zeng R, Zhou Q, Zhang W, Fu X, Wu Q, Lu Y, Shi J, Zhou S (2019) Icariin-mediated activation of autophagy confers protective effect on rotenone induced neurotoxicity in vivo and in vitro. Toxicol Rep 6:637–644. https://doi.org/10.1016/j.toxrep.2019.06.014
In S, Hong CW, Choi B, Jang BG, Kim MJ (2016) Inhibition of mitochondrial clearance and Cu/Zn-SOD activity enhance 6-hydroxydopamine-induced neuronal apoptosis. Mol Neurobiol 53(1):777–791. https://doi.org/10.1007/s12035-014-9087-9
Achour I, Arel-Dubeau AM, Renaud J, Legrand M, Attard E, Germain M, Martinoli MG (2016) Oleuropein prevents neuronal death, mitigates mitochondrial superoxide production and modulates autophagy in a dopaminergic cellular model. Int J Mol Sci 17(8):1293. https://doi.org/10.3390/ijms17081293
Lin TK, Chen SD, Chuang YC, Lin HY, Huang CR, Chuang JH, Wang PW, Huang ST et al (2014) Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int J Mol Sci 15(1):1625–1646. https://doi.org/10.3390/ijms15011625
Zaman V, Shields DC, Shams R, Drasites KP, Matzelle D, Haque A, Banik NL (2021) Cellular and molecular pathophysiology in the progression of Parkinson’s disease. Metab Brain Dis 36(5):815–827. https://doi.org/10.1007/s11011-021-00689-5
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491. https://doi.org/10.3233/JPD-130230
Lin CS, Lee SH, Huang HS, Chen YS, Ma MC (2015) H2O2 generated by NADPH oxidase 4 contributes to transient receptor potential vanilloid 1 channel-mediated mechanosensation in the rat kidney. Am J Physiol Renal Physiol 309(4):F369-376. https://doi.org/10.1152/ajprenal.00462.2014
Gough DR, Cotter TG (2011) Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2(10):e213. https://doi.org/10.1038/cddis.2011.96
Singh K, Maity P, Krug L, Meyer P, Treiber N, Lucas T, Basu A, Kochanek S et al (2015) Superoxide anion radicals induce IGF-1 resistance through concomitant activation of PTP1B and PTEN. EMBO Mol Med 7(1):59–77. https://doi.org/10.15252/emmm.201404082
Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277(23):20336–20342. https://doi.org/10.1074/jbc.M111899200
Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A 101(47):16419–16424. https://doi.org/10.1073/pnas.0407396101
Sato S, Furuya N (2018) Induction of PINK1/Parkin-mediated mitophagy. Methods Mol Biol 1759:9–17. https://doi.org/10.1007/7651_2017_7
Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M et al (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI3K-Akt signalling. Nat Cell Biol 8(8):834–842. https://doi.org/10.1038/ncb1441
Kovalchuke L, Mosharov EV, Levy OA, Greene LA (2019) Stress-induced phospho-ubiquitin formation causes parkin degradation. Sci Rep 9(1):11682. https://doi.org/10.1038/s41598-019-47952-5
Panicker N, Kam TI, Wang H, Neifert S, Chou SC, Kumar M, Brahmachari S, Jhaldiyal A et al (2022) Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron 110(15):2422-2437 e2429. https://doi.org/10.1016/j.neuron.2022.05.009
Lonskaya I, Hebron ML, Algarzae NK, Desforges N, Moussa CE (2013) Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson’s disease. Neuroscience 232:90–105. https://doi.org/10.1016/j.neuroscience.2012.12.018
Funding
This work was supported in part by the grants from the National Natural Science Foundation of China (Nos. 81873781, 81271416, 82101337), National Institutes of Health (CA115414), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD-14KJB180010), BSKY Scientific Research from Anhui Medical University (XJ201813), and American Cancer Society (RSG-08–135-01-CNE).
Author information
Authors and Affiliations
Contributions
LC and SH conceived the project. QY, SH, and LC designed the experiments. QY, RZ, TL, and LY performed the experiments. QY, RZ, SH, and LC analyzed the data. ZZ, LH, WW, RZ, XC, and YY contributed reagents/materials/analysis tools. QY, RZ, SH, and LC wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
The experiments involving animals in this study were handled in accordance with the guidelines issued by the animal ethics committee (IACUC Certificate No. 200408), and were in compliance with the guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary Fig. S1
Ectopic expression of PTEN-C/S and/or myr-Akt, or myr-Akt and/or pretreatment with rapamycin rescues from PD toxins-induced reduction of autophagosomes in neuronal cells. PC12 cells, infected with Ad-PTEN-C/S, Ad-myr-Akt and/or Ad-LacZ (as control) and infected with/without Ad-GFP-LC3, respectively, were treated with/without 6-OHDA (120 μM), MPP+ (1 mM) or rotenone (1 μM) for 24 h, or pretreated with/without rapamycin (100 ng/ml) for 2 h and then treated with/without 6-OHDA, MPP+ or rotenone for 24 h. A and B Representative GFP-LC3 puncta imaging (in green) in the cells was shown by using GFP-LC3 assay. Scale bar: 2 μm. (PNG 448 kb)

Supplementary Fig. S2
CAT blocks PD toxins-induced diminishment of autophagosomes in neuronal cells. PC12, SH-SY5Y cells and primary neurons were pretreated with/without CAT (350 U/ml) for 1 h and then exposed to 6-OHDA (120 μM), MPP+ (1 mM) or rotenone (1 μM) for 24 h. Afterwards, representative GFP-LC3 puncta (in green) in the cells was detected using GFP-LC3 assay. Scale bar: 2 μm. (PNG 184 kb)

Supplementary Fig. S3
Mito-TEMPO attenuates PD toxins-induced generation of H2O2 and decrease of autophagosomes in neuronal cells. PC12, SH-SY5Y cells and primary neurons were pretreated with/without Mito-TEMPO (10 μM) for 1 h and then exposed to 6-OHDA (120 μM), MPP+ (1 mM) or rotenone (1 μM) for 24 h. A Representative intracellular H2O2 (in green) was detected using a peroxide-selective probe H2DCFDA. Scale bar: 20 μm. B Representative GFP-LC3 puncta imaging (in green) in the cells was shown by using GFP-LC3 assay. Scale bar: 2 μm. (PNG 388 kb)

Supplementary Fig. S4
Silencing Parkin confers resistance to PD toxins-evoked decrease of autophagosomes in neuronal cells. PC12 cells, infected with lentiviral shRNA to Parkin or GFP (as control) and infected with/without Ad-GFP-LC3, respectively, were treated with/without 6-OHDA (120 μM), MPP+ (1 mM) or rotenone (1 μM) for 24 h. Afterwards, representative GFP-LC3 puncta (in green) in the cells was detected using GFP-LC3 assay. Scale bar: 2 μm. (PNG 78 kb)

Supplementary Fig. S5
Overexpression of Parkin reinforces PD toxins-induced loss of autophagosomes in neuronal cells. PC12 cells, infected with lentiviral FLAG-Parkin or EGFP (as control) and infected with/without Ad-GFP-LC3, respectively, were treated with/without 6-OHDA (120 μM), MPP+ (1 mM) or rotenone (1 μM) for 24 h. Afterwards, representative GFP-LC3 puncta (in green) in the cells was detected using GFP-LC3 assay. Scale bar: 2 μm. (PNG 79 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Q., Zhang, R., Li, T. et al. Mitochondrial Hydrogen Peroxide Activates PTEN and Inactivates Akt Leading to Autophagy Inhibition-Dependent Cell Death in Neuronal Models of Parkinson’s Disease. Mol Neurobiol 60, 3345–3364 (2023). https://doi.org/10.1007/s12035-023-03286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03286-y