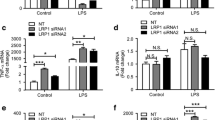
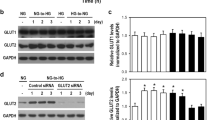
Abstract
M1/M2 polarization transitions of microglial phenotypes determine the states of neuroinflammation, which is critical in the pathophysiology of diabetic encephalopathy. This study aims to investigate the effects of advanced glycation end products (AGEs) on the microglial polarization state, the role of miR-146a-5p in the regulation of microglial polarization, and the underlying signaling pathways. BV-2 cells were incubated with N-ε-carboxymethyl lysine (CML), one kind of Advanced Glycation End Products (AGEs), to induce polarization. CD11b and iNOS and CD206 and Arg-1 were used to evaluate M1 and M2 microglia, respectively. The mRNA and protein expression levels of miR-146a-5p, transcription factor NF-κB, and inflammasome NLRP3 were measured. High and low expression of miR-146a-5p in the BV-2 cell line was generated by lentivirus transfection technology. RAGE, TLR-4, and NF-κB antagonists were applied to evaluate the underlying signaling pathways. Compared with the control group, CML upregulated the M1 phenotype and downregulated the M2 phenotype. These effects were reversed by overexpression of miR-146a. Furthermore, the expression of inflammasome NLRP3 and NF-κB was upregulated in the CML group and was reduced after miR-146a overexpression. And then overexpression of miR-146a effects was reversed by inhibition miR-146a expression. An NF-κB antagonist (PDTC), a RAGE antagonist (FPS-ZMI), and a TLR-4 antagonist (TLI-095) all reversed the polarization state induced by CML. In summary, CML induced polarization transitions to M1 phenotype and promoted inflammasome NLRP3 expression in BV-2 cells. The RAGE or TLR-4/miR-146a/NLRP3/NF-кB pathway might participate in the regulation of CML-induced BV-2 polarization.








Similar content being viewed by others
Data Availability
All relevant data is contained within the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- APS:
-
Ammonium persulfate
- BSA:
-
Albumin from bovine serum
- BCA:
-
Bicinchoninic acid
- BV-2:
-
BV-2 microglia
- CML:
-
Nε-carboxymethyl-lysine
- CAS:
-
Chinese Academy of Sciences
- CDNA:
-
Complementary DNA
- DEPC:
-
Diethyl pyrocarbonate
- DMSO:
-
Dimethyl sulfoxide
- ECL:
-
Enhanced chemiluminescence
- FBS:
-
Fetal bovine serum
- GFP:
-
Green fluorescence protein
- MOI:
-
Multiplicity of infection
- PCR:
-
Polymerase chain reaction
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBS:
-
Phosphate-buffered solution
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PVDF:
-
Polyvinylidene fluoride
- RPM:
-
Revolutions per minute
- RPMI:
-
RPMI Medium 1640 basic (1 ×)
- RAGE:
-
Receptors of advanced glycation end products
- SDS:
-
Sodium dodecyl sulfate
- TBS:
-
Tris-buffered saline
- TEMED:
-
Tetraaminoethylenediamine
- TLR-4:
-
Toll-like receptor-4
References
Cukierman T, Gerstein HC, Williamson JD (2005) Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 48:2460–2469
Lila C, Delia R, Daniela D et al (2017) Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases [J]. Mediators Inflamm 2017:5048616
Uesugi N, Sakata N, Nagai R, Jono T, Horiuchi S, Takebayashi S (2000) Glycoxidative modification of AA amyloid deposits in renal tissue. Nephrol Dial Transplant 15:355–365
Bar KJ, Franke S, Wenda B, Muller S, Kientsch-Engel R, Stein G et al (2003) Pentosidine and n(epsilon)-(carboxymethyl)-lysine in Alzheimer’s disease and vascular dementia. Neurobiol Aging 24:333–338
Marchand F, Perretti M, McMahon SB (2005) Role of the immune system in chronic pain. Nat Rev Neurosci 6:521–532
Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14:591–604
Shi S, Yin HJ, Li J, Wang L, Wang WP, Wang XL (2020) Studies of pathology and pharmacology of diabetic encephalopathy with KK-Ay mouse model. CNS Neurosci Ther 26:332–342
Su XQ, Wang XY, Gong FT, Feng M, Bai JJ, Zhang RR et al (2020) Oral treatment with glycyrrhizin inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization after traumatic spinal cord injury. Brain Res Bull 158:1–8
Ward R, Li W, Abdul Y, Jackson L, Dong G, Jamil S et al (2019) NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res 142:237–250
Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X (2018) Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules 23:522
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A et al (2017) Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Parkinsons Dis 3:30
Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T et al (2015) NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol 18:pyv006
Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X (2016) NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun 56:175–186
Watanabe M, Toyomura T, Mori S (2022) Regulation of inflammatory response based on interaction among AGEs, DAMPs, and/or cytokines [J]. Nihon Yakurigaku Zasshi 157:429–433
Niwa T (2001) Dialysis-related amyloidosis: pathogenesis focusing on age modification. Semin Dial 14:123–126
Cho HJ, Xie C, Cai H (2018) Age-induced neuronal cell death is enhanced in G2019S LRRK2 mutation with increased RAGE expression. Transl Neurodegener 7:1
Dukic-Stefanovic S, Gasic-Milenkovic J, Deuther-Conrad W, Munch G (2003) Signal transduction pathways in mouse microglia N-11 cells activated by advanced glycation endproducts (AGEs). J Neurochem 87:44–55
Lue LF, Kuo YM, Beach T, Walker DG (2010) Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol Neurobiol 41:115–128
Cheray M, Joseph B (2018) Epigenetics control microglia plasticity. Front Cell Neurosci 12:243
Datta M, Staszewski O, Raschi E, Frosch M, Hagemeyer N, Tay TL et al (2018) Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48:514–529
Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39:3–18
Dai Y, Wang S, Chang S, Ren D, Shali S, Li C et al (2020) M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol 142:65–79
Hu L, Zhang H, Wang B, Ao Q, He Z (2020) MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int Immunopharmacol 80:106141
Devaraj S, Tobias P, Jialal I (2011) Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine 55:441–445
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Hayden MR (2019) Type 2 diabetes mellitus increases the risk of late-onset Alzheimer’s disease: ultrastructural remodeling of the neurovascular unit and diabetic gliopathy. Brain Sci 9:262
Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z (2016) The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med 7:57
Hwang IK, Choi JH, Nam SM, Park OK, Yoo DY, Kim W et al (2014) Activation of microglia and induction of pro-inflammatory cytokines in the hippocampus of type 2 diabetic rats. Neurol Res 36:824–832
Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016) High-fructose intake as risk factor for neurodegeneration: key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75
Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W et al (2017) Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6:897–908
Volny O, Kasickova L, Coufalova D, Cimflova P, Novak J (2015) MicroRNAs in cerebrovascular disease. Adv Exp Med Biol 888:155–195
Sadlon A, Takousis P, Alexopoulos P, Evangelou E, Prokopenko I, Perneczky R (2019) MiRNAs identify shared pathways in Alzheimer’s and Parkinson’s diseases. Trends Mol Med 25:662–672
Ma F, Zhang X, Yin KJ (2020) MicroRNAs in central nervous system diseases: a prospective role in regulating blood-brain barrier integrity. Exp Neurol 323:113094
Juzwik CA, Drake SS, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A et al (2019) MicroRNA dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol 182:101664
Li L, Chen XP, Li YJ (2010) MicroRNA-146a and human disease. Scand J Immunol 71:227–231
Luo Q, Feng Y, Xie Y, Shao Y, Wu M, Deng X et al (2019) Nanoparticle-microRNA-146a-5p polyplexes ameliorate diabetic peripheral neuropathy by modulating inflammation and apoptosis. Nanomedicine 17:188–197
Liu XS, Fan B, Szalad A, Jia L, Wang L, Wang X et al (2017) MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes 66:3111–3121
Wang Y, Ma WQ, Zhu Y, Han XQ, Liu N (2018) Exosomes derived from mesenchymal stromal cells pretreated with advanced glycation end product-bovine serum albumin inhibit calcification of vascular smooth muscle cells. Front Endocrinol (Lausanne) 9:524
Kubota K, Nakano M, Kobayashi E, Mizue Y, Chikenji T, Otani M et al (2018) An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS ONE 13:e0204252
Li X, Ji Z, Li S, Sun YN, Liu J, Liu Y et al (2015) MiR-146a-5p antagonized AGEs- and p.G-LPS-induced ABCA1 and ABCG1 dysregulation in macrophages via IRAK-1 downregulation. Inflammation 38:1761–1768
Ghosh S, Castillo E, Frias ES, Swanson RA (2018) Bioenergetic regulation of microglia. Glia 66(6):1200–1212. https://doi.org/10.1002/glia.23271
Sutterwala FS, Haasken S, Cassel SL (2014) Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 1319:82–95
Ibrahim ZA, Armour CL, Phipps S, Sukkar MB (2013) RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 56:739–744
Shirasuna K, Seno K, Ohtsu A, Shiratsuki S, Ohkuchi A, Suzuki H et al (2016) AGEs and HMGB1 increase inflammatory cytokine production from human placental cells, resulting in an enhancement of monocyte migration. Am J Reprod Immunol 75:557–568
Watanabe M, Toyomura T, Wake H, Liu K, Teshigawara K, Takahashi H et al (2020) Differential contribution of possible pattern-recognition receptors to advanced glycation end product-induced cellular responses in macrophage-like RAW264.7 cells. Biotechnol Appl Biochem 67:265–272
Cao X, Xia Y, Zeng M, Wang W, He Y, Liu J (2019) Caffeic acid inhibits the formation of advanced glycation end products (AGEs) and mitigates the ages-induced oxidative stress and inflammation reaction in human umbilical vein endothelial cells (HUVECs). Chem Biodivers 16:e1900174
Yeh WJ, Yang HY, Pai MH, Wu CH, Chen JR (2017) Long-term administration of advanced glycation end-product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J Nutr Biochem 39:68–76
Hong J, Li G, Zhang Q, Ritter J, Li W, Li PL (2019) D-ribose induces podocyte NLRP3 inflammasome activation and glomerular injury via AGEs/RAGE pathway. Front Cell Dev Biol 7:259
Dai J, Chen H, Chai Y (2019) Advanced glycation end products (AGEs) induce apoptosis of fibroblasts by activation of NLRP3 inflammasome via reactive oxygen species (ROS) signaling pathway. Med Sci Monit 25:7499–7508
Yi X, Zhang L, Lu W, Tan X, Yue J, Wang P et al (2019) The effect of NLRP inflammasome on the regulation of AGEs-induced inflammatory response in human periodontal ligament cells. J Periodontal Res 54:681–689
Kong X, Lu AL, Yao XM, Hua Q, Li XY, Qin L et al (2017) Activation of NLRP3 inflammasome by advanced glycation end products promotes pancreatic islet damage. Oxid Med Cell Longev 2017:9692546
Jia HL, Lu CQ, Wang J, Ren DD, Chen YQ (2019) The effect of interference of NLRP3 with shRNA on ages-induced inflammatory response in myocardial cell. Sichuan Da Xue Xue Bao Yi Xue Ban 50:7–12
Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B et al (2012) A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122:1377–1392
Funding
Funding for this work was supported by the National Natural Science Foundation of China (NSFC No.82200871,82270873), Natural Science Foundation of Fujian Province (2020J01221, 2020J01226), Medical Innovation Project of Fujian Province (2020CXA044), Science Fund for Distinguished Young Scholars of Fujian Province (2020GGA057), and the Postdoctoral Start-up fund of the Second Affiliated Hospital of Fujian Medical University (2020BSH02).
Author information
Authors and Affiliations
Contributions
Yinqiong Huang and Xiaoyun Lin contributed equally to this manuscript. Yinqiong Huang, Xiaoyun Lin, and Xiahong Lin conceptualized and designed these studies, performed them, and wrote the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study is only cell culture research. No ethics approval is required in this study.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Lin, X. & Lin, X. MiR-146a-5p Contributes to Microglial Polarization Transitions Associated With AGEs. Mol Neurobiol 60, 3020–3033 (2023). https://doi.org/10.1007/s12035-023-03252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03252-8