Abstract
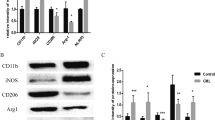
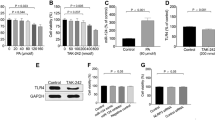
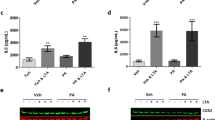
Protein phosphatase 2A (PP2A), the activity of which is dictated by the composition of its regulatory subunit, is strongly related to the progression of neurodegenerative disease. The potential role of PP2A on the phenotypic transition of microglial cells under obese conditions is poorly explored. An understanding of the role of PP2A and identification of regulatory subunits contributing to microglial phenotypic transitions in obese condition may serve as a therapeutic target for obesity-associated neurodegeneration. C57BL/6 mice were exposed to obese-associated vascular dementia conditions by performing unilateral common carotid artery occlusion on obese mice of microglial polarization and PP2A activity using flow cytometry, real-time PCR, western blotting, and immunoprecipitation enzymatic assay, followed identifications of PP2A regulatory subunits using LCMS and RT-PCR. Chronic HFD feeding significantly increased the populations of infiltrated macrophages, showing a high percentage of CD86+ in VaD mice, and the expression of pro-inflammatory cytokines, and we observed that PP2A modulates metabolic reprogramming of microglia by regulating OXPHOS/ECAR activity. Using Co-IP and LCMS, we identified the six specific regulatory subunits, namely PPP2R2A, PPP2R2D, PPP2R5B, PPP2R5C, PPP2R5D, and PPP2R5E, that are associated with microglial-activation during obesity-associated-VaD. Interestingly, pharmacological up-regulation of PP2A more significantly suppressed the expression of TNF-alpha than other pro-inflammatory-cytokines and increased the expression of Arginase-1, suggesting that PP2A modulates microglial-phenotypic transitions through TNF-α/Arg-1 axis. Our present findings demonstrate microglial polarization in HFD associated with VaD, and point towards a therapeutic target by providing specific PP2A regulatory-subunits implicated in microglial activation during obesity-related-vascular-dementia.






Similar content being viewed by others
Data Availability
This article contains all of the data that were generated/analyzed during this investigation.
References
Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10:217–224. https://doi.org/10.1038/nrneurol.2014.38
Kim J, Yoon N, Jin S, Diano S (2019) Microglial UCP2 Mediates inflammation and obesity induced by high-fat feeding. Cell Metab 30(5):952-962.e5. https://doi.org/10.1007/978-1-4939-8994-2_1
Contu L, Nizari S, Heath C, and Hawkes C (2019). Pre- and post-natal high fat feeding differentially affects the structure and integrity of the neurovascular unit of 16-month old male and female mice. Frontiers in Neuroscience 13. https://doi.org/10.3389/fnins.2019.01045
Spencer S, Basri B, Sominsky L, Soch A, Ayala M, Reineck P, Gibson B, Barrientos R (2019) High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol Aging 74:121–134. https://doi.org/10.1016/j.neurobiolaging.2018.10.018
Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala E, Tuomilehto J et al (2005) Obesity and vascular risk factors at midlife and the risk of dementia and alzheimer disease. Arch Neurol 62(10). https://doi.org/10.1001/archneur.62.10.1556
Lecordier S, Manrique-Castano D, El Moghrabi Y, El Ali A (2021) Neurovascular alterations in vascular dementia: emphasis on risk factors. Front Aging Neurosc 13. Published 2021 Sep 10. https://doi.org/10.3389/fnagi.2021.727590
Javadpour P, Dargahi L, Ahmadiani A, Ghasemi R (2019) To be or not to be: PP2A as a dual player in CNS functions, its role in neurodegeneration, and its interaction with brain insulin signaling. Cell Mol Life Sci 76(12):2277–2297. https://doi.org/10.1007/s00018-019-03063-y
Leong W, Xu W, Wang B, Gao S, Zhai X, Wang C, Gilson E, Ye J, Lu Y (2020) PP2A subunit PPP2R2C is downregulated in the brains of Alzheimer’s transgenic mice. Aging 12(8):6880–6890
Sontag J, Sontag E (2014) Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front Mol Neurosci 7. https://doi.org/10.3389/fnmol.2014.00016
Yu U, Yoo B, Ahn J (2014) Regulatory B subunits of protein phosphatase 2A are involved in site-specific regulation of Tau protein phosphorylation. Korean J Physiol Pharmacol 18(2):155. https://doi.org/10.4196/kjpp.2014.18.2.155
Mazhar S, Taylor S, Sangodkar J, Narla G (2019) Targeting PP2A in cancer: combination therapies. Biochimica et Biophysica Acta (BBA) - Mole Cell Res 186(1):51–63. https://doi.org/10.1016/j.bbamcr.2018.08.020
Jiwa N, Garrard P, Hainsworth A (2010) Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem 115(4):814–828. https://doi.org/10.1111/j.1471-4159.2010.06958.x
Lee J, Tansey M (2013) Microglia isolation from adult mouse brain. Methods Mol Biol 2013(1041):17–23. https://doi.org/10.1007/978-1-62703-520-0_3
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1(6):2856–2860. https://doi.org/10.1038/nprot.2006.468
Korrodi-Gregório L, Silva J, Santos-Sousa L, Freitas M, Felgueiras J, Fardilha M (2014) TGF-β cascade regulation by PPP1 and its interactors –impact on prostate cancer development and therapy. J Cell Mol Med 18(4):555–567. https://doi.org/10.1111/jcmm.12266
Morikawa M, Derynck R, Miyazono K (2016) TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8(5):a021873. https://doi.org/10.1101/cshperspect.a021873
Li Y, Shen X, Li L, Zhao T, Bernstein K, Johnson A, Lyden P, Fang J, Shi P (2017) Brain transforming growth factor-β resists hypertension via regulating microglial activation. Stroke 48(9):2557–2564. https://doi.org/10.1161/STROKEAHA.117.017370
Hong S, Kang M, Lee K, and Yu K (2016). High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci Rep 6(1). https://doi.org/10.1038/srep30265
Arnold S, Lucki I, Brookshire B, Carlson G, Browne C, Kazi H, Bang S, Choi B et al (2014) High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67:79–87. https://doi.org/10.1016/j.nbd.2014.03.011
Cristobal I, Cirauqui C, Castello-Cros R, Garcia-Orti L, Calasanz M, Odero M (2013) Downregulation of PPP2R5E is a common event in acute myeloid leukemia that affects the oncogenic potential of leukemic cells. Haematologica 98(9):e103–e104. https://doi.org/10.3324/haematol.2013.084731
Cheng Y, Seibert O, Klöting N, Dietrich A, Straßburger K, Fernández-Veledo S, Vendrell J, Zorzano A et al (2015) PPP2R5C couples hepatic glucose and lipid homeostasis. PLOS Genetics 11(10):e1005561. https://doi.org/10.1371/journal.pgen.1005561
Biswas D, Cary W, Nolta J (2020) PPP2R5D-related intellectual disability and neurodevelopmental delay: a review of the current understanding of the genetics and biochemical basis of the disorder. Int J Mol Sci 21(4):1286. https://doi.org/10.3390/ijms21041286
Shiwa M, Yoneda M, Okubo H, Ohno H, Kobuke K, Monzen Y, Kishimoto R, Nakatsu Y et al (2015) Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLOS ONE 10(8):e0135554. https://doi.org/10.1371/journal.pone.0135554
Louis J, Martens E, Borghgraef P, Lambrecht C, Sents W, Longin S, Zwaenepoel K, Pijnenborg R et al (2011) Mice lacking phosphatase PP2A subunit PR61/B’δ ( Ppp2r5d ) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3β. Proc Natl Acad Sci 108(17):6957–6962. https://doi.org/10.1073/pnas.1018777108
Montagne A, Nation D, Zlokovic B (2020) APOE4 accelerates development of dementia after stroke. Stroke 51(3):699–700. https://doi.org/10.1161/STROKEAHA.119.028814
Theendakara V, Bredesen D, Rao R (2017) Downregulation of protein phosphatase 2A by apolipoprotein E: implications for Alzheimer’s disease. Mol Cell Neurosci 83:83–91. https://doi.org/10.1016/j.mcn.2017.07.002
Koren-Iton A, Salomon-Zimri S, Smolar A, Shavit-Stein E, Dori A, Chapman J, Michaelson D (2020) Central and peripheral mechanisms in ApoE4-driven diabetic pathology. Int J Mol Sci 21(4):1289. https://doi.org/10.3390/ijms21041289
Schleicher U, Paduch K, Debus A, Obermeyer S, König T, Kling J, Ribechini E, Dudziak D et al (2016) TNF-mediated restriction of Arginase 1 expression in myeloid cells triggers type 2 NO synthase activity at the site of infection. Cell Rep 15(5):1062–1075. https://doi.org/10.1016/j.celrep.2016.04.001
Yu Z, Fukushima H, Ono C, Sakai M, Kasahara Y, Kikuchi Y, Gunawansa N, Takahashi Y et al (2017) Microglial production of TNF-alpha is a key element of sustained fear memory. Brain Behav Immun 59:313–321. https://doi.org/10.1016/j.bbi.2016.08.011
Nematullah M, Hoda M, Nimker S, Khan F (2020) Restoration of PP2A levels in inflamed microglial cells: important for neuroprotective M2 microglial viability. Toxicol Appl Pharmacol 409:115294. https://doi.org/10.1016/j.taap.2020.115294
Yin J, Li R, Liu W, Chen Y, Zhang X, Li X, He X, Duan C (2018) Neuroprotective effect of protein phosphatase 2A/tristetraprolin following subarachnoid hemorrhage in rats. Front Neurosci 12. https://doi.org/10.3389/fnins.2018.00096
Sangodkar J, Farrington C, McClinch K, Galsky M, Kastrinsky D, Narla G (2015) All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase. FEBS J 283(6):1004–1024. https://doi.org/10.1111/febs.13573
Seshacharyulu P, Pandey P, Datta K, Batra S (2013) Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett 335(1):9–18. https://doi.org/10.1016/j.canlet.2013.02.036
Nematullah M, Hoda MN, Khan F (2018) Protein phosphatase 2A: a double-faced phosphatase of cellular system and its role in neurodegenerative disorders. Mol Neurobiol 55(2):1750–1761. https://doi.org/10.1007/s12035-017-0444-3
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12. https://doi.org/10.3389/fncel.2018.00488
Lull M, Block M (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365. https://doi.org/10.1016/j.nurt.2010.05.014
Hu X, Li P, Guo Y, Wang H, Leak R, Chen S, Gao Y, Chen J (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070. https://doi.org/10.1161/STROKEAHA.112.659656
Qin C, Fan W, Liu Q, Shang K, Murugan M, Wu L, Wang W, Tian D (2017) Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48(12):3336–3346
Acknowledgements
MN acknowledges the fellowships provided by Maulana Azad National Fellowship, University Grants Commission, India.
Author information
Authors and Affiliations
Contributions
M.N. and F.K. conceptualized and designed the experiments. M.N., F.R., and S.N. performed the experiments. M.N., F.K., and F.R. analyzed the data. M.N. and F.K. wrote the manuscript. The overall work was done under the supervision of F.K.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
The animal experimental procedures were approved by the Institutional Animal Ethics Committee (IAEC) of Jamia Hamdard, following the guidelines of The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (registration number: 173/GO/ReBi/S/2000/CPCSEA; project proposal number 1732). This study met the ethical requirements of animal experiments.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nematullah, M., Rashid, F., Nimker, S. et al. Protein Phosphatase 2A Regulates Phenotypic and Metabolic Alteration of Microglia Cells in HFD-Associated Vascular Dementia Mice via TNF-α/Arg-1 Axis. Mol Neurobiol 60, 4049–4063 (2023). https://doi.org/10.1007/s12035-023-03324-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03324-9