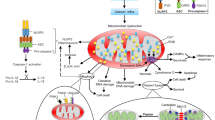
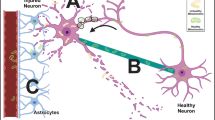
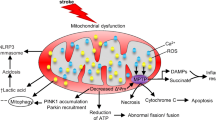
Abstract
Regardless of the progress made in the pathogenesis of ischemic stroke, it remains a leading cause of adult disability and death. To date, the most effective treatment for ischemic stroke is the timely recanalization of the occluded artery. However, the short time window and reperfusion injury have greatly limited its application and efficacy. Mitochondrial dysfunction and ATP depletion have become regarded as being hallmarks of neuropathophysiology following ischemic stroke. Mitochondrial transplantation is a novel potential therapeutic intervention for ischemic stroke that has sparked widespread concern during the past few years. This review summarizes and discusses the effects of mitochondrial transplantation in in vitro and in vivo ischemic stroke models. In addition, pharmacological interventions promoting mitochondrial transplantation are reviewed and discussed. We also discuss the potential challenges to the clinical application of mitochondrial transplantation in the treatment of ischemic stroke.


Similar content being viewed by others
Data Availability
Not applicable.
References
Nian K, Harding IC, Herman IM, Ebong EE (2020) Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front Physiol 11:605398. https://doi.org/10.3389/fphys.2020.605398
Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L (2022) Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology 98(3):e279–e290. https://doi.org/10.1212/wnl.0000000000013115
Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(9995):743–800. https://doi.org/10.1016/S0140-6736(15)60692-4
Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, Vivanco-Hidalgo RM, Ois A, Rodríguez-Campello A, Cuadrado-Godia E, Sayols-Baixeras S, Elosua R, Roquer J, Jiménez-Conde J (2016) Ischemic stroke patients are biologically older than their chronological age. Aging (Albany NY) 8(11):2655–2666. https://doi.org/10.18632/aging.101028
Terni E, Giannini N, Brondi M, Montano V, Bonuccelli U, Mancuso M (2015) Genetics of ischaemic stroke in young adults. BBA Clin 3:96–106. https://doi.org/10.1016/j.bbacli.2014.12.004
Feske SK (2021) Ischemic Stroke. Am J Med 134(12):1457–1464. https://doi.org/10.1016/j.amjmed.2021.07.027
Barthels D (1866) Das H (2020) Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis 4:165260. https://doi.org/10.1016/j.bbadis.2018.09.012
Gauberti M, Lapergue B, Martinez de Lizarrondo S, Vivien D, Richard S, Bracard S, Piotin M, Gory B (2018) Ischemia-reperfusion injury after endovascular thrombectomy for ischemic stroke. Stroke 49(12):3071–3074. https://doi.org/10.1161/strokeaha.118.022015
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/b978-0-12-394309-5.00006-7
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England J Med 359(13):1317–1329. https://doi.org/10.1056/NEJMoa0804656
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738. https://doi.org/10.1016/j.cmet.2011.08.016
Alle H, Roth A, Geiger JR (2009) Energy-efficient action potentials in hippocampal mossy fibers. Science (New York, NY) 325(5946):1405–1408. https://doi.org/10.1126/science.1174331
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science (New York, NY) 305(5684):626–629. https://doi.org/10.1126/science.1099320
Jordan J, de Groot PW, Galindo MF (2011) Mitochondria: the headquarters in ischemia-induced neuronal death. Cent Nerv Syst Agents Med Chem 11(2):98–106. https://doi.org/10.2174/187152411796011358
Orellana-Urzúa S, Rojas I, Líbano L, Rodrigo R (2020) Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des 26(34):4246–4260. https://doi.org/10.2174/1381612826666200708133912
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. https://doi.org/10.1016/j.pneurobio.2013.11.006
Yang JL, Mukda S, Chen SD (2018) Diverse roles of mitochondria in ischemic stroke. Redox Biol 16:263–275. https://doi.org/10.1016/j.redox.2018.03.002
Singh A, Faccenda D, Campanella M (2021) Pharmacological advances in mitochondrial therapy EBioMedicine 65:103244. https://doi.org/10.1016/j.ebiom.2021.103244
Garone C, Viscomi C (2018) Towards a therapy for mitochondrial disease: an update. Biochem Soc Trans 46(5):1247–1261. https://doi.org/10.1042/bst20180134
Russell OM, Gorman GS, Lightowlers RN, Turnbull DM (2020) Mitochondrial diseases: hope for the future. Cell 181(1):168–188. https://doi.org/10.1016/j.cell.2020.02.051
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. https://doi.org/10.1038/nature18928
McCully JD, Levitsky S, Del Nido PJ, Cowan DB (2016) Mitochondrial transplantation for therapeutic use. Clin Transl Med 5(1):16. https://doi.org/10.1186/s40169-016-0095-4
Park A, Oh M, Lee SJ, Oh K-J, Lee E-W, Lee SC, Bae K-H, Han BS, Kim WK (2021) Mitochondrial transplantation as a novel therapeutic strategy for mitochondrial diseases. Int J Mol Sci 22(9):4793. https://doi.org/10.3390/ijms22094793
Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng Y, Shi L, Meloni BP, Zhang C, Zheng M, Gao J (2021) Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther 6(1):65. https://doi.org/10.1038/s41392-020-00440-z
Huang T, Zhang T, Gao J (2022) Targeted mitochondrial delivery: a therapeutic new era for disease treatment. J Control Release 343:89–106. https://doi.org/10.1016/j.jconrel.2022.01.025
Chen W, Huang J, Hu Y, Khoshnam SE, Sarkaki A (2020) Mitochondrial transfer as a therapeutic strategy against ischemic stroke. Transl Stroke Res 11(6):1214–1228. https://doi.org/10.1007/s12975-020-00828-7
Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018) Mitochondria in ischemic stroke: new insight and implications. Aging Dis 9(5):924–937. https://doi.org/10.14336/ad.2017.1126
Lin L, Wang X, Yu Z (2016) Ischemia-reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem Pharmacol (Los Angel) 5(4):213–228. https://doi.org/10.4172/2167-0501.1000213
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord: Drug Targets 12(5):698–714. https://doi.org/10.2174/1871527311312050015
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4(6):461–470. https://doi.org/10.1111/j.1747-4949.2009.00387.x
Jelinek M, Jurajda M, Duris K (2021) Oxidative stress in the brain: basic concepts and treatment strategies in stroke. Antioxidants (Basel) 10(12):1886. https://doi.org/10.3390/antiox10121886
Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C (2007) Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci 27(27):7083–7093. https://doi.org/10.1523/jneurosci.1645-07.2007
Rothman SM, Olney JW (1986) Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol 19(2):105–111. https://doi.org/10.1002/ana.410190202
Shen Z, Xiang M, Chen C, Ding F, Wang Y, Shang C, Xin L, Zhang Y, Cui X (2022) Glutamate excitotoxicity: potential therapeutic target for ischemic stroke. Biomed Pharmacother 151:113125. https://doi.org/10.1016/j.biopha.2022.113125
Kaplan-Arabaci O, Acari A, Ciftci P, Gozuacik D (2022) Glutamate scavenging as a neuroreparative strategy in ischemic stroke. Front Pharmacol 13:866738. https://doi.org/10.3389/fphar.2022.866738
Luoma JI, Kelley BG, Mermelstein PG (2011) Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids 76(9):845–855. https://doi.org/10.1016/j.steroids.2011.02.013
Rossi DJ, Oshima T, Attwell D (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403(6767):316–321. https://doi.org/10.1038/35002090
Papazian I, Kyrargyri V, Evangelidou M, Voulgari-Kokota A, Probert L (2018) Mesenchymal stem cell protection of neurons against glutamate excitotoxicity involves reduction of NMDA-triggered calcium responses and surface GluR1, and is partly mediated by TNF. Int J Mol Sci 19(3):651. https://doi.org/10.3390/ijms19030651
Verma M, Wills Z, Chu CT (2018) Excitatory dendritic mitochondrial calcium toxicity: implications for parkinson’s and other neurodegenerative diseases. Front Neurosci 12:523. https://doi.org/10.3389/fnins.2018.00523
Bauer TM, Murphy E (2020) Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 126(2):280–293. https://doi.org/10.1161/circresaha.119.316306
Carinci M, Vezzani B, Patergnani S, Ludewig P, Lessmann K, Magnus T, Casetta I, Pugliatti M, Pinton P, Giorgi C (2021) Different roles of mitochondria in cell death and inflammation: focusing on mitochondrial quality control in ischemic stroke and reperfusion. Biomedicines 9(2):169. https://doi.org/10.3390/biomedicines9020169
Alishahi M, Farzaneh M, Ghaedrahmati F, Nejabatdoust A, Sarkaki A, Khoshnam SE (2019) NLRP3 inflammasome in ischemic stroke: as possible therapeutic target. Int J Stroke 14(6):574–591. https://doi.org/10.1177/1747493019841242
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD (2013) Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191(10):5230–5238. https://doi.org/10.4049/jimmunol.1301490
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36(3):401–414. https://doi.org/10.1016/j.immuni.2012.01.009
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464(7285):104–107. https://doi.org/10.1038/nature08780
Zhang JZ, Liu Z, Liu J, Ren JX, Sun TS (2014) Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue. Int J Mol Med 33(4):817–824. https://doi.org/10.3892/ijmm.2014.1650
Tian H, Chen X, Liao J, Yang T, Cheng S, Mei Z, Ge J (2022) Mitochondrial quality control in stroke: From the mechanisms to therapeutic potentials. J Cell Mol Med 26(4):1000–1012. https://doi.org/10.1111/jcmm.17189
Stotland A (1853) Gottlieb RA (2015) Mitochondrial quality control: Easy come, easy go. Biochim Biophys Acta 1853(10 Pt B):2802–2811. https://doi.org/10.1016/j.bbamcr.2014.12.041
Wu M, Gu X, Ma Z (2021) Mitochondrial quality control in cerebral ischemia-reperfusion injury. Mol Neurobiol 58(10):5253–5271. https://doi.org/10.1007/s12035-021-02494-8
Yang M, He Y, Deng S, Xiao L, Tian M, Xin Y, Lu C, Zhao F, Gong Y (2021) Mitochondrial quality control: a pathophysiological mechanism and therapeutic target for stroke. Front Mol Neurosci 14:786099. https://doi.org/10.3389/fnmol.2021.786099
Clark MA, Shay JW (1982) Mitochondrial transformation of mammalian cells. Nature 295(5850):605–607. https://doi.org/10.1038/295605a0
Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103(5):1283–1288. https://doi.org/10.1073/pnas.0510511103
McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S (2009) Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296(1):H94-h105. https://doi.org/10.1152/ajpheart.00567.2008
Ikeda G, Santoso MR, Tada Y, Li AM, Vaskova E, Jung JH, O’Brien C, Egan E, Ye J, Yang PC (2021) Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol 77(8):1073–1088. https://doi.org/10.1016/j.jacc.2020.12.060
Doulamis IP, Guariento A, Duignan T, Kido T, Orfany A, Saeed MY, Weixler VH, Blitzer D, Shin B, Snay ER, Inkster JA, Packard AB, Zurakowski D, Rousselle T, Bajwa A, Parikh SM, Stillman IE, Del Nido PJ, McCully JD (2020) Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol 319(3):F403-f413. https://doi.org/10.1152/ajprenal.00255.2020
Nascimento-Dos-Santos G, de-Souza-Ferreira E, Linden R, Galina A, Petrs-Silva H (2021) Mitotherapy: unraveling a promising treatment for disorders of the central nervous system and other systemic conditions. Cells 10(7):1827. https://doi.org/10.3390/cells10071827
Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P (2021) Mitochondrial transfer in cancer: a comprehensive review. Int J Mol Sci 22(6):3245. https://doi.org/10.3390/ijms22063245
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18(5):759–765. https://doi.org/10.1038/nm.2736
Xing C, Lo EH (2017) Help-me signaling: non-cell autonomous mechanisms of neuroprotection and neurorecovery. Prog Neurobiol 152:181–199. https://doi.org/10.1016/j.pneurobio.2016.04.004
Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, Dubois-Randé JL, Rodriguez AM (2011) Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29(5):812–824. https://doi.org/10.1002/stem.632
Vallabhaneni KC, Haller H, Dumler I (2012) Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev 21(17):3104–3113. https://doi.org/10.1089/scd.2011.0691
Sun C, Liu X, Wang B, Wang Z, Liu Y, Di C, Si J, Li H, Wu Q, Xu D, Li J, Li G, Wang Y, Wang F, Zhang H (2019) Endocytosis-mediated mitochondrial transplantation: transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 9(12):3595–3607. https://doi.org/10.7150/thno.33100
Yamada Y, Ito M, Arai M, Hibino M, Tsujioka T, Harashima H (2020) Challenges in promoting mitochondrial transplantation therapy. Int J Mol Sci 21(17):6365. https://doi.org/10.3390/ijms21176365
Chang JC, Wu SL, Liu KH, Chen YH, Chuang CS, Cheng FC, Su HL, Wei YH, Kuo SJ, Liu CS (2016) Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res 170:40-56.e43. https://doi.org/10.1016/j.trsl.2015.12.003
Paliwal S, Chaudhuri R, Agrawal A, Mohanty S (2018) Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 25(1):31. https://doi.org/10.1186/s12929-018-0429-1
D’Souza A, Burch A, Dave KM, Sreeram A, Reynolds MJ, Dobbins DX, Kamte YS, Zhao W, Sabatelle C, Joy GM, Soman V, Chandran UR, Shiva SS, Quillinan N, Herson PS, Manickam DS (2021) Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J Control Release 338:505–526. https://doi.org/10.1016/j.jconrel.2021.08.038
Veziroglu EM, Mias GI (2020) Characterizing extracellular vesicles and their diverse RNA contents. Front Genet 11:700. https://doi.org/10.3389/fgene.2020.00700
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G, Gao W, Li Y, Wang Q (2020) Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci (Lond) 134(16):2161–2175. https://doi.org/10.1042/cs20200530
Shi C, Guo H, Liu X (2021) Platelet mitochondria transplantation rescues hypoxia/reoxygenation-induced mitochondrial dysfunction and neuronal cell death involving the FUNDC2/PIP3/Akt/FOXO3a Axis. Cell Transplant 30:9636897211024210. https://doi.org/10.1177/09636897211024210
Sommer CJ (2017) Ischemic stroke: experimental models and reality. Acta Neuropathol 133(2):245–261. https://doi.org/10.1007/s00401-017-1667-0
Holloway PM, Gavins FN (2016) Modeling ischemic stroke in vitro: status quo and future perspectives. Stroke 47(2):561–569. https://doi.org/10.1161/strokeaha.115.011932
Goldberg MP, Choi DW (1993) Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci 13(8):3510–3524. https://doi.org/10.1523/jneurosci.13-08-03510.1993
Xie Q, Zeng J, Zheng Y, Li T, Ren J, Chen K, Zhang Q, Xie R, Xu F, Zhu J (2021) Mitochondrial transplantation attenuates cerebral ischemia-reperfusion injury: possible involvement of mitochondrial component separation. Oxid Med Cell Longev 2021:1006636. https://doi.org/10.1155/2021/1006636
Huang PJ, Kuo CC, Lee HC, Shen CI, Cheng FC, Wu SF, Chang JC, Pan HC, Lin SZ, Liu CS, Su HL (2016) Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transplant 25(5):913–927. https://doi.org/10.3727/096368915x689785
Li X, Li Y, Zhang Z, Bian Q, Gao Z, Zhang S (2021) Mild hypothermia facilitates mitochondrial transfer from astrocytes to injured neurons during oxygen-glucose deprivation/reoxygenation. Neurosci Lett 756:135940. https://doi.org/10.1016/j.neulet.2021.135940
Kalda A, Eriste E, Vassiljev V, Zharkovsky A (1998) Medium transitory oxygen-glucose deprivation induced both apoptosis and necrosis in cerebellar granule cells. Neurosci Lett 240(1):21–24. https://doi.org/10.1016/s0304-3940(97)00914-2
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, Wang Q (2019) Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res 356:322–331. https://doi.org/10.1016/j.bbr.2018.09.005
Liu K, Guo L, Zhou Z, Pan M, Yan C (2019) Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res 123:74–80. https://doi.org/10.1016/j.mvr.2019.01.001
Yip HK, Dubey NK, Lin KC, Sung PH, Chiang JY, Chu YC, Huang CR, Chen YL, Deng YH, Cheng HC, Deng WP (2021) Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube-mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed Pharmacother 139:111593. https://doi.org/10.1016/j.biopha.2021.111593
Pourmohammadi-Bejarpasi Z, Roushandeh AM, Saberi A, Rostami MK, Toosi SMR, Jahanian-Najafabadi A, Tomita K, Kuwahara Y, Sato T, Roudkenar MH (2020) Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res Bull 165:70–80. https://doi.org/10.1016/j.brainresbull.2020.09.018
Kim JY, Park J, Chang JY, Kim SH, Lee JE (2016) Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp Neurobiol 25(5):241–251. https://doi.org/10.5607/en.2016.25.5.241
Xu S, Lu J, Shao A, Zhang JH, Zhang J (2020) Glial cells: role of the immune response in ischemic stroke. Front Immunol 11:294. https://doi.org/10.3389/fimmu.2020.00294
Oo TT, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2020) Potential Roles of myeloid differentiation factor 2 on neuroinflammation and its possible interventions. Mol Neurobiol 57(11):4825–4844. https://doi.org/10.1007/s12035-020-02066-2
Nakamura Y, Lo EH, Hayakawa K (2020) Placental mitochondria therapy for cerebral ischemia-reperfusion injury in mice. Stroke 51(10):3142–3146. https://doi.org/10.1161/strokeaha.120.030152
Babenko VA, Silachev DN, Zorova LD, Pevzner IB, Khutornenko AA, Plotnikov EY, Sukhikh GT, Zorov DB (2015) Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: The role of crosstalk between cells. Stem Cells Transl Med 4(9):1011–1020. https://doi.org/10.5966/sctm.2015-0010
Bertero E, Maack C, O’Rourke B (2018) Mitochondrial transplantation in humans: “magical” cure or cause for concern? J Clin Investig 128(12):5191–5194. https://doi.org/10.1172/jci124944
Nasoni MG, Carloni S, Canonico B, Burattini S, Cesarini E, Papa S, Pagliarini M, Ambrogini P, Balduini W, Luchetti F (2021) Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J Pineal Res 71(1):e12747. https://doi.org/10.1111/jpi.12747
Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR (2015) Melatonin and brain inflammaging. Prog Neurobiol 127–128:46–63. https://doi.org/10.1016/j.pneurobio.2015.02.001
Fiorina P, Lattuada G, Silvestrini C, Ponari O, Dall’Aglio P (1999) Disruption of nocturnal melatonin rhythm and immunological involvement in ischaemic stroke patients. Scand J Immunol 50(2):228–231. https://doi.org/10.1046/j.1365-3083.1999.00579.x
Atanassova PA, Terzieva DD, Dimitrov BD (2009) Impaired nocturnal melatonin in acute phase of ischaemic stroke: cross-sectional matched case-control analysis. J Neuroendocrinol 21(7):657–663. https://doi.org/10.1111/j.1365-2826.2009.01881.x
Bernardi P, Rasola A, Forte M, Lippe G (2015) The Mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95(4):1111–1155. https://doi.org/10.1152/physrev.00001.2015
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED (2021) Cardiac energy metabolism in heart failure. Circ Res 128(10):1487–1513. https://doi.org/10.1161/circresaha.121.318241
Acknowledgements
All figures are created with BioRender.com.
Funding
This work was supported by the Senior Research Scholar Grant from the National Research Council of Thailand (SCC); Thailand Science Research and Innovation-Chaing Mai University (Fundamental Fund 2565 to SCC); the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC); and the Chiang Mai University Center of Excellence Award (NC).
Author information
Authors and Affiliations
Contributions
NC and SCC: conception and funding acquisition; HTH: writing-original draft; TOO, NA, NC, and SCC: writing-reviewing and editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
All authors have given final approval of this version and agreed to publish this article here.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Oo, T.T., Apaijai, N. et al. An Updated Review of Mitochondrial Transplantation as a Potential Therapeutic Strategy Against Cerebral Ischemia and Cerebral Ischemia/Reperfusion Injury. Mol Neurobiol 60, 1865–1883 (2023). https://doi.org/10.1007/s12035-022-03200-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03200-y