Abstract
Musashi RNA-binding proteins (MSIs) retain a pivotal role in stem cell maintenance, tumorigenesis, and nervous system development. Recently, we showed in C. elegans that Musashi (MSI-1) actively promotes forgetting upon associative learning via a 3’UTR-dependent translational expression of the Arp2/3 actin branching complex. Here, we investigated the evolutionary conserved role of MSI proteins and the effect of their pharmacological inhibition on memory. Expression of human Musashi 1 (MSI1) and Musashi 2 (MSI2) under the endogenous Musashi promoter fully rescued the phenotype of msi-1(lf) worms. Furthermore, pharmacological inhibition of human MSI1 and MSI2 activity using (-)- gossypol resulted in improved memory retention, without causing locomotor, chemotactic, or learning deficits. No drug effect was observed in msi-1(lf) treated worms. Using Western blotting and confocal microscopy, we found no changes in MSI-1 protein abundance following (-)- gossypol treatment, suggesting that Musashi gene expression remains unaltered and that the compound exerts its inhibitory effect post-translationally. Additionally, (-)- gossypol suppressed the previously seen rescue of the msi-1(lf) phenotype in worms expressing human MSI1 specifically in the AVA neuron, indicating that (-)- gossypol can regulate the Musashi pathway in a memory-related neuronal circuit in worms. Finally, treating aged worms with (-)- gossypol reversed physiological age-dependent memory decline. Taken together, our findings indicate that pharmacological inhibition of Musashi might represent a promising approach for memory modulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Learning and memory constitute cognitive functions comprising a wide variety of complex components. Even though there has been a lot of effort to elucidate the molecular aspects of memory [1,2,3], actively controlled forgetting is an equally important but much less investigated mechanism [4,5,6]. We have recently demonstrated in C. elegans that the sole Musashi RNA-binding protein (RBP) plays an important regulatory role in forgetting by modulating cytoskeletal changes at the translational level [7]. The Musashi protein was originally discovered as a key player in asymmetric cell division, stem cell function, and cell fate determination in Drosophila [8]. In vertebrates, the two MSI orthologues, Musashi1 (MSI1) and Musashi2 (MSI2), are mostly expressed in stem cells. In particular, MSI1 is a marker of neural progenitor cells [9], and outside the nervous system can be found in the stem cells of the gut [10] and epithelial cells of the mammary gland [11]. MSI2 is also present in differentiated neurons of the adult brain as well as in hematopoietic stem cells [12]. Expression of MSI1 has been correlated with the grade of malignancy and proliferative activity in gliomas and melanomas, whilst mutations in MSI2 have been associated with poor prognosis in certain types of cancers. Both MSI1 and MSI2 harbor two tandem RNA recognition motifs (RRMs) located at their N-terminal region, followed by a putative disordered region. The amino acid sequences of RRM1 and RRM2 of MSI1 exhibit 85% identity to those of MSI2 respectively, suggesting that the two proteins might target some mRNAs in a similar fashion. Indeed, an in vitro study identified the UAG RNA sequence as the core MSI1 binding motif [13], while recent genome-wide analyses of MSI2 targets revealed binding of MSI2 to mRNA 3’UTRs that contain multiple copies of UAG motifs as well as polyU pentamers [14, 15].
Dysregulated expression of RBPs can be detected in various human diseases ranging from neurological disorders to cancer [16, 17], rendering them potential targets for therapeutic intervention. However, identifying compounds that are able to disrupt protein-RNA interactions remains an arduous task due to the complexity of targeting RBPs or the selection of their interacting mRNAs. Previous studies have shed light on small molecule inhibitors of MSI1 in an effort to reduce Musashi-mediated tumourigenesis. Ω-9 fatty acids, such as oleic acid and its derivatives, were identified as allosteric inhibitors of MSI1 RNA-binding activity [18], while a fluorescence polarization (FP) competition assay revealed that (-)- gossypol, a natural compound extracted from cottonseed, acts as an in vitro inhibitor of MSI1 by recognizing and directly binding to its RNA-binding domain 1 (RBD1) [19].
Caenorhabditis elegans represents a powerful model organism to elucidate the molecular mechanisms of learning and memory. It is characterized by a simple nervous system of 302 neurons capable of exhibiting behavioral plasticity towards an array of stimuli [20, 21]. In C. elegans, the sole Musashi ortholog (msi-1) has been implicated in male mating [22]. We previously demonstrated that MSI-1 actively regulates forgetting via translational repression of actin branching complexes [7]. (-)- gossypol was the first small molecule compound to exhibit potent anticancer activity in vivo by disrupting MSI1 activity via the Notch/Wnt pathway [19], rendering it an ideal candidate to test for its potential effects on memory. In the current study, we investigated the evolutionary conserved role of MSI proteins and the effect of their pharmacological inhibition on memory and physiological age-dependent memory decline in these animals.
Methods
General Methods and Strains Used
Common reagents were obtained from Sigma (Sigma-Aldrich, St Louis, MO) unless otherwise indicated. Standard methods were used for maintaining and manipulating C. elegans [23]. All experiments were conducted using a synchronized population of young adult hermaphrodites. The C. elegans Bristol strain, variety N2, was used as the wild-type reference strain in all experiments. Alleles and transgenes used were the following: msi-1(os1), msi-1(os1);utrIs15[pmsi-1::human msi1cDNA::msi-1 3’UTR, psur-5::mDsred], msi-1(os1);utrIs14[pmsi-1::human msi2cDNA::msi1 3’UTR, psur-5::mDsred], msi-1(os1);utrIs40[prig-3::human msi1cDNA::msi-1 3’UTR, psur-5::mDsred], msi-1 (utr55[YPET::3xFLAG]), utrSi43[prig-3::LoxP::BFP::LoxP::FLP-DS::SL2::GFP::H2B],unc-119(ed3), msi-1(utr55[YPET::3xFLAG]);utrSi43[prig-3::LoxP::BFP::LoxP::FLP-DS::SL2::GFP::H2B],unc-119(ed3), utrIs11[pmsi-1::GFP::arx-2 3’UTR, punc-119 +].
Extrachromosomal transgenic lines were generated by injecting DNA at a concentration of 100 ng/μl into both arms of the syncytial gonad of worms as previously described (Mello et al., 1991). psur-5::mDsRed was used as a transformation marker at a concentration of 10 ng/μl. Chromosomal integration of extrachromosomal arrays was done by UV radiation for 12 s or 15 s. Following integration, generated strains were backcrossed four times to the wild-type strain.
C. elegans Behavior Assays
Chemotaxis to different compounds was assessed in synchronized young adult worms as described previously [24]. Briefly, a population of well-fed, young adults was washed three times with CTX buffer (5 mM KH2PO4/K2HPO4 pH 6.0, 1 mM CaCl2, and 1 mM MgSO4) and 100–200 worms were placed in the center of a 10-cm CTX test plate. Worms were given a choice between a spot of attractant diluted in ethanol with 20 mM sodium-azide and a counter spot with ethanol and sodium-azide (NaN3). The distribution of the worms over the plate was determined after 1 h and the chemotaxis index (CI) was calculated as previously described (Chemotaxis Index = \(\frac{\mathrm{no of worms in attractant }-\mathrm{no of worms incontrol}}{\mathrm{total amount of worms on plate}}\)) [24]. Olfactory conditioning was assessed as previously described [25], with a few modifications. Starvation conditioning was performed without food in the presence of 2 μl undiluted diacetyl (DA) spotted on the plate for 1 h on 10 cm CTX plates (5 mM KH2PO4/K2HPO4 pH 6.0, 1 mM CaCl2, 1 mM MgSO4, 2% agar). Following conditioning, worms were tested for their chemotaxis towards DA immediately after or after 1 h rest to assess short-term associative memory.
Long-term associative memory was induced with two cycles of conditioning with DA with 30-min rest between trainings. Following conditioning, worms were kept on NGM plates in the presence of abundant food for 24 h and tested for chemotaxis to DA after the recovery phase [26].
Pharmacological treatment was performed by soaking worms for 2 h in CTX supplemented with either dimethyl sulfoxide (DMSO) alone or the indicated concentration of gossypol (AT101, Sigma-Aldrich) in DMSO, prior to olfactory conditioning. Gossypol was dissolved in absolute DMSO to make a 10 mM stock solution. After a 2-h incubation with the drug, worms were washed 3 times with CTX and tested for their preference to DA immediately (naïve), after conditioning, 1 h (STAM) or 24 h (LTAM) after conditioning. Furthermore, any potential delayed drug toxicity was tested 2 h and 24 h after treatment with the drug by assessing naïve chemotaxis to DA.
Locomotory Rate Assays
Locomotory rate assays were carried out to test for any gossypol-related effects on worm motility. After treatment, worms were allowed to recover for 30 min on plates seeded with OP50. Individual worms were transferred on 6 cm assay plates, and allowed to move freely for 3 min and then the number of body bends of fifteen animals from each strain and treatment group as indicated was counted for 1 min.
Molecular Biology
Rescue of the msi-1(lf) phenotype was performed with a 12.9-kb construct comprised of a 7.7 kb fragment of the C. elegans msi-1 promoter region, the full-length msi1 or msi2 human cDNA designed with NcoI and KpnI compatible ends, fused with a 1.1 kb fragment of the C. elegans msi-1 3’UTR. Full-length human msi1 and msi2 cDNA were amplified from the MGC clones pENTR™223.1 and pOTB7 respectively (Transomic Technologies, Huntsville, AL, USA). Primers used for human msi1 cDNA amplification were as follows: CCA TGG AGA CTG ACG CGC CCC AG and GGT ACC TCA GTG GTA CCC ATT GGT GAA GG and for human msi2 cDNA amplification CCA TGG AGG CAA ATG GGA GCC AAG and GGT ACC TCA ATG GTA TCC ATT TGT AAA GG. The thermal cycle was programmed for 120 s at 95 °C as initial denaturation, followed by 30 cycles of 30 s at 95 °C for denaturation, 30 s at 64 °C as annealing, 90 s at 72 °C for extension, and final extension at 72 °C for 5 min. For the tissue-specific rescue experiments, the full-length msi1 or msi2 human cDNA and the msi-1 3’UTR were fused to a 3 kb fragment of the rig-3 promoter using NEBuilder Hifi DNA assembly (New England Biolabs, Ipswich, MA).
Reverse Transcription PCR
Total RNA was isolated from synchronized adult wild-type, hmsi1 and hmsi2 transgene-carrying animals using a Direct-zol RNA MiniPrep kit (Zymo Research Cooperation) with DNase treatment. RNA integrity was measured using the Agilent 2100 Bioanalyzer (Agilent Genomics, California, USA). cDNA was synthesized using SuperScript™ III First-Strand Synthesis System (ThermoFischer Scientific, MA, USA) according to the manufacturer’s recommendations using 1 µg of purified RNA. Primers designed to amplify human Musashi cDNA were the following: for msi-1 ATT GAC CCT AAG GTG GCC TTC C and TGG CGG CGC TGA TGT AAC TG and for msi-2 ACG TTC GCA GAC CCA GCA AG and TGG GAA GCC TGG GAA CTG ATA G. Plasmid DNA was used to test for primer annealing. To avoid amplification from contaminating genomic DNA, two sets of primers per construct were designed to span the endogenous C. elegans msi1 promoter and human cDNA boundary region (hmsi1: ACG AAA CAA ACC ATG GAG AC and TGC GTA GTC TGC CAA CTG AG; CAA ACC ATG GAG ACT GAC GC and GAA GTA TTC GCG CAG CCC TT hmsi2: ACG AAA CAA ACC ATG GAG GCA and GGC TAT CTG GTG AGG TCT GC; CAA ACC ATG GAG GCA AAT GGG and CTA AGG CTA TCT GGT GAG GTC. Actin was used as a control for cDNA amplification with the following primers (forward primer: GCC CAA TCC AAG AGA GGT ATC C, reverse primer: GGC AAC ACG AAG CTC ATT G). Thermal cycle was programmed for 120 s at 95 °C as initial denaturation, followed by 30 cycles of 30 s at 95 °C for denaturation, 30 s at 60 °C as annealing, 60 s at 72 °C for extension, and final extension at 72 °C for 5 min.
Targeted Modification of msi-1 Using CRISPR/Cas9
Endogenous tagging of msi-1 with YPET::3xFlag was generated as described previously [27]. 601 bp and 722 bp homology arms flanking the N-terminus of msi-1 were PCR amplified from N2 genomic DNA and inserted into the mNG^SEC&3xFlag vector pDD283 using NEBuilder Hifi DNA assembly (New England Biolabs, Ipswich, MA). The Cas9 target site was selected using the Sequence Scan for CRISPR database (http://cistrome.org/SSC/) and inserted into pDD162 [28]. The sgRNA sequence used was 5’- ATGACAACGACAGTATCAACGTTTTAGAGCTAGAAATAGCAAGT-3’. A mixture of 50 ng/µl Cas9–sgRNA plasmid, 10 ng/µl repair template, and 2.5 ng/µl pCFJ90, 5 ng/µl pCFJ104 and 10 ng/µl sur-5p::dsRed co-injection markers were injected into the gonads of young adults [29]. The knock-in line was established, the SEC cassette was excised using heat shock and the msi-1 YPET::3xFlag line was sequenced to verify the correct insertion of the tag.
Western Blot Analysis
For protein extraction, 50–100 worms per condition were lysed directly in ice-cold 3xLaemmli buffer (6%w/v SDS, 30%glycerol, 120 mM Tris–Cl pH 6.8, 0.03% w/v bromophenol blue) and heated for 5 min at 95 °C. Samples were directly subjected to SDS-PAGE, transferred to PVDF membranes, blocked with 5% nonfat dry milk in TBST (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20) and incubated with primary antibodies as indicated. Antibodies used were mouse anti-FLAG (1:2000, Sigma Aldrich, St. Louis, MI) and mouse anti-tubulin (1:20,000, Merck Millipore, Burlington, MA). Primary antibodies were detected using HRP-coupled secondary antibodies (1:10,000, Jackson ImmunoResearch Laboratories, Cambridge House, UK). The chemiluminescent signal was developed using Clarity and ClarityMax Western Blotting Substrates (BioRad Laboratories Inc., Hercules, CA) followed by detection with a FujiFilm ImageQuant LAS-4000 detector (GE Healthcare, Chicago, IL).
Fluorescence Microscopy
Whole worms were mounted on 3% agarose pads and immobilized with 0.5% NaN3. Synchronized 1-day-old adult worms were imaged using a Zeiss LSM 880 inverted scanning confocal microscope equipped with 25 × and 63 × oil immersion objectives. All recorded images were processed and quantified using Fiji software (version 2.1.0/1.53c). Fluorescence in AVA was measured using the integrated density function and the corresponding background signal was subtracted. Due to the dense neuronal signal in the head of the animal, AVA was first localized and selected in the BFP channel and the identical selection was then superimposed onto the YPET channel.
Statistics
All data and statistical analyses were carried out using the Prism software (version: 9.1.2). Main effects and interaction terms were tested using ANOVA. Statistical tests for significance were done with F-tests using sum-of-squares type I. The p-value threshold was set to nominal significance (p < 0.05). In case of a significant main or interaction effect, the significance between data was tested using post hoc t-tests. P-values of the post hoc tests were corrected for the number of tests calculated per analysis (Bonferroni-correction per analysis: pbonf < 0.05). For the imaging quantification, a two-tailed unpaired Student’s t-test was carried out to assess any differences between groups and the p-value threshold was set to nominal significance (p < 0.05). All figures were created using Adobe Illustrator (version 24.3.2).
Results
Human MSI1 and MSI2 Proteins Are Implicated in Memory
Previously, we have shown that the sole C. elegans Musashi ortholog, msi-1, actively promotes forgetting [7]. To study the potentially conserved role of MSIs in memory, we performed rescue experiments with the human Musashi homologues MSI1 (Is[hsmi1 +]) and MSI2 (Is[hsmi2 +]) in C. elegans, using established context-dependent associative learning and memory assays [25, 26].
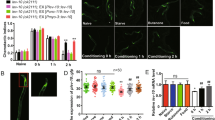
In order to study the functional conservation of Musashi proteins between worms and humans (Fig. 1a), we generated transgenic C. elegans lines expressing human MSI1 and MSI2 in a msi-1 loss of function (lf) background. The msi1(os1) allele used in this study is a 1.6 kb deletion in the msi-1 gene that results in a severely truncated protein that has no RRMs and is likely nonfunctional [22]. The transgenic animals were created by fusing 7.7 kb of the C. elegans msi-1 promoter region to either the human MSI1 or MSI2 cDNA, followed by 1.1 kb of the C. elegans msi-1 3’UTR. Following integration of the extrachromosomal arrays, expression of both transgenes was confirmed with RT-PCR (Fig. 1b) and the lines were tested in a short- and long-term negative olfactory learning and memory paradigm. Initially, wild type, msi-1(lf), msi-1(lf); Is[hsmi1 +], and msi-1(lf); Is[hsmi2 +] animals were tested for their chemotaxis toward diacetyl (DA) prior to and following aversive associative learning (conditioning). All animals exhibited similar chemotaxis, independent of the genotype tested (Fig. 2a-d), suggesting that ectopic expression of human MSIs in worms does not influence baseline chemotaxis or associative learning. Moreover, we investigated the role of human MSI1 and MSI2 in short-term [STAM] and long-term [LTAM] associative memory. In the case of STAM, worms were subjected to one round of conditioning and tested for memory performance following a 1-h recovery period [25]. As previously shown, msi-1(lf) exhibited increased memory retention in short-term memory [7], a phenotype which was fully restored to wild-type levels in msi-1(lf) mutants carrying either the human MSI1 or MSI2 transgene (Fig. 2a-b). Similar to short-term memory, expression of either human MSIs fully rescued the effect of msi-1(lf) mutant worms on memory retention after a 24-h delay period (Fig. 2c-d) [7, 26]. Altogether, our results indicate that both human Musashi homologues are able to replace C.elegans MSI-1 protein function in STAM as well as in LTAM.
Sequence alignment of C. elegans and human Musashi orthologues and validation of transgene expression. (a) Grey boxes indicate identical amino acids and green boxes show similar amino acids. The two RNA-binding domains (RRM1 and RRM2), showing the highest conservation amongst species, are underlined. (b) Expression of human MSI1 and MSI2 was validated using cDNA extracted from the transgenic lines. N2 cDNA was used as a negative control for Musashi expression and the original plasmids used for cloning were used as positive controls for primer annealing. Actin was used as a further control for cDNA integrity. Primers annealing to the endogenous msi-1 promoter/ human cDNA boundary identifies any amplification coming from contaminating genomic DNA (“primers genomic DNA”) and primers binding within the human cDNA region illustrate true expression (“primers cDNA”)
Human MSI1 and MSI2 are involved in the regulation of memory loss. (a, b) Negative STAM was tested in wild-type, msi-1(lf) and msi-1(lf) mutant worms rescued with the human MSI1 or MSI2 construct. Worms were assayed toward DA without (naïve), with preincubation with DA and starvation (cond) or after 1 h (1 h delay). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for hMSI1: F(4, 153) = 10.675, p = 1.17 × 10–7, interaction effect for hMSI2: F(4, 288) = 10.9514, p = 2.79 × 10–8) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (c, d) Negative LTAM in the different genotypes was tested following two consecutive conditioning phases and DA preference was tested immediately (cond) and after a 24 h (24 h delay) recovery period. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for hMSI1: F(4, 153) = 3.663, p = 7.03 × 10–3, interaction effect for hMSI2: F(4, 207) = 3.63507, p = 6.92 × 10–3) and post hoc t-tests as indicated (Bonferroni's adjusted p values are reported). All experiments were done in triplicates and repeated at least five times
Human MSI1 Protein Is Necessary in the AVA Interneuron
Previously, it was shown that expression of the C. elegans MSI-1 under the rig-3 promoter, which drives expression primarily in the AVA interneuron, was sufficient to rescue the memory phenotype of msi-1(lf) mutants [7]. The AVA interneuron is located in the lateral ganglia of the head of the worm and is one of four bilaterally symmetric interneuron pairs (AVA, AVB, AVD, and PVC). It receives input from several upstream neurons (e.g., ASH, PVD, AFD) and sends out axons that run the entire length of the ventral nerve cord, providing input to neurons such as A-type (VA, DA) and AS motor neurons. AVA functions as a driver cell for backward locomotion (reversal) and was previously shown to play an important role in aversive long-term memory [7, 26]. To find out whether human MSI1 also shows a similar tissue-specific function as its C.elegans ortholog, we performed a rescue experiment by expressing the human MSI1 cDNA specifically under the control of the C. elegans rig-3 promoter in a msi-1(lf) background. As a result, we could observe rescue in both the STAM (Fig. 3a) as well as in the LTAM (Fig. 3b) testing paradigm suggesting that expression only in AVA is sufficient to restore the phenotype to wild-type.
Human MSI1 regulates memory loss in the AVA interneuron. (a) Negative STAM was tested in wild-type, msi-1(lf) and msi-1(lf) mutant worms expressing the human MSI1 construct. Worms were assayed toward DA without (naïve), with preincubation with DA and starvation (cond) or after 1 h (1 h delay). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect: F(4, 126) = 3.423, p = 1.07 × 10–2) and post hoc t-tests as indicated (Bonferroni's adjusted p values are reported). (b) Negative LTAM in the different genotypes was tested following two consecutive conditioning phases and DA preference was tested immediately (cond) and after a 24 h (24 h delay) recovery period. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect: F(4, 153) = 2.538, p = 4.22 × 10–2) and post hoc t-tests as indicated (Bonferroni's adjusted p values are reported). All experiments were done in triplicates and repeated at least five times
(-)- Gossypol Has No Adverse Effect on the Chemotactic or Locomotory Behavior of C. elegans
Surface plasmon and nuclear magnetic resonance studies have demonstrated that (-)- gossypol directly interacts with the RNA-binding groove of MSI1 and disrupts binding to its downstream targets [19]. (-)- Gossypol’s therapeutic applicability has been limited due to the toxicity it harbors [30]. To address any potential toxic effects, we first measured the baseline chemotactic response of worms towards diacetyl following treatment with different (-)- gossypol concentrations (1–10 μΜ). In both human MSI1 and MSI2 transgenic worms, we observed no significant immediate (Fig. 4a-b) or delayed changes (Fig. 4c-d) in the chemotaxis index of worms treated with (-)- gossypol compared to untreated DMSO controls.
(-)- gossypol harbors no immediate or delayed toxicity. (a, b) Naïve chemotaxis was tested in msi-1(lf) mutant worms rescued with the human MSI1 construct following a (a) 2-h and (b) 24-h delay, post- (-)- gossypol treatment. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with one-way ANOVA (2 h delay effect: F(4, 70) = 0.5650, p = 0.6888, 24 h delay effect: F(4, 55) = 0.3248, p = 0.86) and post hoc t-tests as indicated (Bonferroni's adjusted p values are reported) ns, not significant. (c, d) Naïve chemotaxis was tested in msi-1(lf) mutant worms rescued with the human MSI2 construct following a (c) 2-h and (d) 24-h delay post- (-)- gossypol treatment. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with one-way ANOVA (2 h delay effect: F(4, 55) = 1.190, p = 0.3256, 24 h delay effect: F(4, 55) = 2.086, p = 0.0951) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported) ns, not significant. All experiments were done in triplicates and repeated at least five times
To determine whether (-)- gossypol modulates the locomotory behavior of C. elegans, we measured the number of body bends per minute in worms treated with DMSO as vehicle control and 10 µM (-)- gossypol. Following a 2-h drug treatment, worms were transferred to clean OP50 seeded plates and allowed to move freely for 3 min before counting the locomotory rate. Using this measure, we found no difference in the locomotor response of C. elegans exposed to 10 µM (-)- gossypol versus the control group (Fig. 5a).
Effects of (-)- gossypol on the motoric and chemosensation behavior of worms. (a) Locomotory rate of DMSO and (-)- gossypol treated msi-1(lf) worms rescued with human MSI1 and human MSI2. Data were analyzed using a two-tailed Student’s test with p < 0.05. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. (b, c) Chemotaxis after (-)- gossypol treatment was measured towards DA as previously described [24] in msi-1(lf) worms rescued with human (b) MSI1 and (c) MSI2. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for hMSI1: F(4, 99) = 0.9442, p = 0.44, interaction effect for hMSI2: F(4, 99) = 2.149, p = 0.08) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported), ns, not significant. (d, e) Chemotaxis after (-)- gossypol treatment was measured towards IAA as previously described [24] in msi-1(lf) worms rescued with human (d) MSI1 and (e) MSI2. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for hMSI1: F(4, 126) = 0.2922, p = 0.88, interaction effect for hMSI2: F(4, 99) = 1.181, p = 0.32) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported), ns, not significant. All experiments were done in triplicates and repeated at least five times
To investigate whether (-)- gossypol influences the chemosensation of worms, we measured the chemotactic response of DMSO-, 10 µM and 50 µM (-)- gossypol- treated worms towards different concentrations of diacetyl (DA) and isoamyl-alcohol (IAA). In msi-1(lf) worms rescued with the human MSI1 construct, (-)- gossypol did not affect chemosensation at any given concentration of drug or chemoattractant (Fig. 5b, d). In msi-1(lf) worms rescued with the human MSI2 construct, we observed a reduction in the chemotaxis towards the lowest DA concentration (10–4) tested when worms were treated with 10 µM of (-)- gossypol (Fig. 5c). In contrast to DA, a high concentration of (-)- gossypol (50 µM) treatment significantly reduced the chemotactic response of msi-1(os1);utrIs14[pmsi-1::human msi2, psur-5::dsred] worms towards 10–3 and 10–4 IAA concentrations (Fig. 5e), suggesting that higher (-)- gossypol concentrations can impair the ability of these worms to detect low concentrations of odors. It is important to note that DA and IAA are detected via the AWA and AWC amphid sensory neurons respectively [24]; therefore, (-)- gossypol likely affects chemosensation independent of the sensory neuron implicated.
(-)- Gossypol Inhibits Musashi-Mediated Forgetting and Improves Short- and Long-Term Memory
Even though it is widely known that (-)- gossypol exhibits potent anti-tumor activity in vivo, no research has been done to shed light on its potential effects on memory. Thus, in order to pharmacologically modify MSI activity and memory, we investigated the possible effect of (-)- gossypol treatment on the memory performance of human MSI1- and MSI2-expressing msi-1(lf) animals. Treating worms with (-)- gossypol for 2 h prior to conditioning induced suppression of the phenotype observed in human MSI1-carrying worms compared to the DMSO treated line, thus improving memory in the STAM and LTAM behavioral assay (Fig. 6a-b). Similar findings were observed in human MSI2 expressing C. elegans lines, where (-)- gossypol inhibited the previously seen rescue of the forgetting defect phenotype, both in a STAM and LTAM behavioral assay (Fig. 6c-d). Thus, (-)- gossypol was able to improve both short- and long-term memory in either of the humanized C.elegans strains.
(-)- gossypol inhibits the memory rescue phenotype of human MSI1 and MSI2. (a, b) msi-1(lf) mutant worms rescued with the human MSI1 construct were tested for negative (a) STAM and (b) LTAM memory performance following a 2-h (-)- gossypol treatment. Worms were assayed toward DA without (naïve) or with preincubation with DA and starvation (cond) and after 1 h (1 h delay). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for STAM: F(2, 84) = 9.2769, p = 2.28 × 10–4, interaction effect for LTAM: F(2, 48) = 5.18113, p = 9.17 × 10–3) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (c, d) msi-1(lf) mutant worms rescued with the human MSI2 construct were tested for negative (c) STAM and (d) LTAM performance following a 2-h (-)- gossypol treatment. Negative LTAM in the different genotypes was tested following two consecutive conditioning phases and DA preference was tested immediately (cond) and after a 24 h (24 h delay) recovery period. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect for STAM: F(2, 100) = 2.756, p = 0.0684, interaction effect for LTAM: F(2, 66) = 19.7209, p = 1.92 × 10–7) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). All experiments were done in triplicates and repeated at least five times
(-)- Gossypol-Induced Memory Improvement Is Likely Mediated Through Neuron-Specific Musashi Inhibition
It was previously shown that (-)- gossypol does not exclusively inhibit MSI1 activity, but can also act on other proteins, such as on the BCL-2 family members [31, 32]. To investigate the specificity of (-)- gossypol on MSI in the regulation of memory, we treated wild-type and msi-1(lf) worms with either DMSO or 10 µM (-)- gossypol and measured the effect on memory after a 24 h delay. While wild-type worms had a significant increase in LTAM retention when compared to their DMSO-treated control counterpart, msi-1(lf) worms displayed no differences between treatment groups (Fig. 7a).
(-)- gossypol acts via the Musashi pathway to inhibit forgetting. (a) Negative LTAM was tested in wild-type and msi-1(lf) worms treated with DMSO and 10 µM (-)- gossypol. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(6, 204) = 3.8519, p = 1.15 × 10–3) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (b) GFP intensity in integrated transgenic worms expressing GFP under a 7.7-kb fragment of the msi-1 promoter, fused to the arx-2 3′ UTR. GFP signal was measured in untreated worms expressing the array in a wild-type and msi1 (lf) background as well as in wild-type worms treated with either DMSO or (-)- gossypol (immediately or 1 h hour after treatment). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with one-way ANOVA (treatment effect F(4, 164) = 20.51, p = 1.01 × 10–13) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (c) Negative STAM was tested in msi-1(lf) mutant worms expressing human MSI1 under the rig-3 promoter, treated with either DMSO and 10 µM (-)- gossypol. Worms were assayed toward DA without (naïve) or with preincubation with DA and starvation (cond) and after 1 h (1 h delay). Box plots for each treatment and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(2, 48) = 4.756, p = 0.0130) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (d) Negative LTAM was tested in msi-1(lf) mutant worms expressing human MSI1 under the rig-3 promoter, treated with either DMSO or 10 µM (-)- gossypol. LTAM was tested following two consecutive conditioning phases and DA preference was tested immediately (cond) and after a 24 h (24 h delay) recovery period. Box plots for each treatment and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(2, 84) = 4.040, p = 0.0211) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). All experiments were done in triplicates and repeated at least five times
Moreover, we investigated whether Musashi downstream targets change upon (-)- gossypol treatment. We focused on changes in the Arp2/3 actin member ACTR2/ARX-2, previously reported to interact with MSI-1 [7, 33] and whose abundance significantly increased in msi-1 (lf)worms [7] and upon (-)- gossypol treatment [33]. We treated worms expressing GFP under the control of the Musashi promoter, fused to the arx-2 3’UTR (pmsi-1::GFP::arx-2 3’UTR) with either DMSO or 10 µM (-)- gossypol for 2 h and quantified GFP levels of head neurons immediately or 1 h after treatment. We also measured GFP signals of the transgene in untreated wild-type and msi-1 (lf) worms as a control. In line with previous results, we observed a highly significant increase in the GFP signal in msi-1 (lf) worms compared to wild-type worms (Fig. 7b). DMSO-treated worms showed similar GFP expression levels to those of wild-type worms suggesting that DMSO treatment alone has no effect on ARX-2 protein levels (Fig. 7b). Wild-type worms treated with 10 µM (-)- gossypol showed a significant increase of GFP expression compared to untreated and DMSO-treated wild-type worms (Fig. 7b), suggesting that a 2-h treatment with (-)- gossypol is sufficient to partially inhibit Musashi and influence expression of its downstream targets.
The msi-1 promoter has been suggested to drive expression in many tissues such as multiple neurons, muscle cells and the intestine (wormbase.org) which can have implications for the drug response. Consequently, we investigated whether (-)- gossypol has a tissue-specific mode of action rather than an off-target effect. We treated msi-1(lf); prig-3::human msi1cDNA::msi-1 3’UTR worms with either DMSO or 10 µM (-)- gossypol and measured the memory phenotype after a 1-h or 24-h delay. In both the STAM (Fig. 7c) and LTAM (Fig. 7d) paradigms tested, (-)- gossypol treatment was sufficient to suppress the msi-1 rescue phenotype previously shown in the AVA interneuron, further validating that the effect of (-)- gossypol on memory is unlikely driven by alternative, off-target pathways.
(-)- Gossypol Treatment Does Not Influence MSI-1 Protein Abundance
To investigate whether any of the effects of (-)- gossypol could be a result of altered protein expression rather than a result of inhibition of protein function, we conducted a time course assay and examined MSI-1 endogenous protein levels after treatment. We first tagged the endogenous gene at the N-terminal end with a yellow fluorescent protein (YPET) and 3xFLAG using CRISPR/Cas9 (Dickinson et al., 2015). To confirm the functional integrity of the tagged protein, we compared the memory performance between wild-type, msi-1(lf) and MSI-1::YPET::3xFLAG animals and found no difference in either STAM or LTAM (Fig. 8a-b).
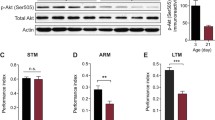
(-)- gossypol treatment does not influence MSI-1 protein abundance. (a) Negative STAM was tested in wild-type, msi-1(lf) and msi-1::3xFLAG::YPET worms. Worms were assayed toward DA without (naïve), with preincubation with DA and starvation (cond) or after 1 h (1 h delay). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(4, 125) = 3.421, p = 0.0109) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (b) Negative LTAM in the different genotypes was tested following two consecutive conditioning phases and DA preference was tested immediately (cond) and after a 24-h (24 h delay) recovery period. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(4, 153) = 3.122, p = 0.0167) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). All experiments were done in triplicates and repeated at least five times. (c) MSI-1 protein abundance following DMSO or (-)- gossypol treatment was analyzed in a time-dependent manner using western blots. For each condition, 50–100 transgenic worms were analyzed using FLAG antibody (top) and as the loading control membranes were reprobed for tubulin (bottom). (d) Relative MSI-1 abundance is plotted at different time points following a 2 h (-)- gossypol treatment and values are presented as a fold change to the DMSO control group. Bars represent mean ± SD from 6 independent blots. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(4, 50) = 0.8197, p = 0.5187), ns, not significant. (e) Representative confocal images of MSI-1 expression in the AVA interneuron (arrows) in MSI-1::YPET::3xFLAG animals. Scale bar, 20 μm. (f) Violin plots displaying quantification of fluorescence intensity in AVA interneuron in MSI-1::YPET::3xFLAG animals at different time points following DMSO and (-)- gossypol treatment. Animals were recorded with identical microscope settings and YPET intensity was measured on z-projected confocal images and quantified using ImageJ software. The center line of the violin plot represents the group median, whereas the bottom and top lines represent the 25th and 75th percentiles respectively. Statistical analysis was performed using a two-way ANOVA (interaction effect F(4, 111) = 0.1538, p = 0.9609), ns, not significant
To check for possible (-)- gossypol-related changes in MSI-1 protein abundance, we treated worms for 2 h with either DMSO or 10 μM gossypol and collected worm fractions at different time points. By carrying out western blot analysis, we could not detect any significant changes in MSI-1 protein levels (Fig. 8c-d), suggesting that (-)- gossypol inhibits MSI-1 function without affecting its abundance. Since the fractions collected are whole worm lysates and represent MSI-1 global expression, we carried out fluorescent microscopy to identify any tissue-specific MSI-1 expression changes. We focused specifically on the AVA neuron and crossed the MSI-1::YPET::3xFLAG strain with an integrated rig-3 promoter-driven BFP line to easily localize the AVA interneuron amongst the dense head neuronal network (Fig. 8e). We could not detect any significant changes in MSI-1 expression at different time points following treatment, further corroborating our western blot results (Fig. 8f).
Effect of msi-1 and (-)- Gossypol on Age-Dependent Cognitive Decline
Physiological, age-dependent memory decline can be readily observed in nematodes with 2-day-old worms already exhibiting a significant decline in olfactory associative memory [34]. To investigate the potential beneficial effect of msi-1 inhibition on age-dependent cognitive decline, we compared LTAM in 1- and 2-day-old wild-type and msi-1 (lf) worms. As expected, the LTAM of wild-type worms significantly decreased with age, whilst msi-1 (lf) displayed no age-dependent memory decline (Fig. 9a). Furthermore, both 1- and 2-day-old msi-1 (lf) worms exhibited a significant increase in LTAM retention compared to their wild-type counterparts. In line with previous results, (-)- gossypol treatment of 2-day-old wild-type worms mimicked the msi-1 (lf) phenotype, with animals displaying improved LTAM retention when compared to their DMSO-treated counterparts (Fig. 9b), suggesting that (-)- gossypol could help improve memory of aged worms.
(-)- Gossypol reverses physiological age-dependent cognitive decline in a MSI-1-dependent manner. (a) Negative LTAM was tested in 1-day and 2-day-old wild-type and msi-1(lf) worms. Worms were assayed toward DA without (naïve), with two rounds of preincubation with DA and starvation (cond) or after 24 h (24 h delay). Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(6, 129) = 11.901 p = 1.33 × 10–10) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). (b) Negative LTAM was assessed in 2-day-old wild-type worms treated with DMSO and 10 µM (-)- gossypol. Box plots for each genotype and condition are presented with whiskers indicating the 10th and 90th percentiles. Statistical significance between groups was assessed with two-way ANOVA (interaction effect F(2, 66) = 14.9156 p = 4.52 × 10–6) and post hoc t-tests as indicated (Bonferroni’s adjusted p values are reported). All experiments were done in triplicates and repeated at least five times
Discussion
A nervous system’s robust cognitive fitness does not stem solely from its ability to form new memories but also from its capacity to erase/forget irrelevant and harmful information [5]. Forgetting should not be regarded as having a negative connotation, but rather be seen as a dynamic cognitive process of the brain, enabling us to adapt and survive in a constantly changing environment. Thus, forgetting constitutes an innate system characterized by its own finely tuned molecular and cellular processes that should be distinguished from those regulating memory encoding, consolidation and retrieval [5].
Here we report that the human Musashi orthologues, MSI1 and MSI2, likely have a conserved role in the regulation of memory. Introducing human MSIs into C. elegans under the control of either the endogenous msi-1 or rig-3 promoter efficiently rescued the forgetting defect of msi-1(lf) worms, both in a short-term and long-term aversive associative learning paradigm. This suggests that despite the differences in the amino acid sequence between worm and human MSI proteins, their function seems to be conserved during the regulation of forgetting. This is the first study to suggest that human MSI proteins could have a role in the active regulation of forgetting. In addition, pharmacological treatment of human MSI1- and MSI2-expressing worms with the natural compound (-)- gossypol, improved memory with no effects on locomotion or memory acquisition of worms. MSI1 and MSI2 display a high degree of sequence and structure similarity and appear to target the same mRNA recognition motif [35]. Therefore, (-)- gossypol could be considered as an MSI1/2 dual inhibitor.
The (-)- gossypol enantiomer used in this study is eliminated slowly [36, 37], constitutes the most biologically active form of the compound and consequently is considered more toxic than (+) gossypol [38]. Toxicity testing assays revealed no immediate or delayed toxic effects of (-)- gossypol concentrations up to 10 µM, however, higher concentrations (50μΜ) exhibited slight toxic effects, as illustrated by the reduced chemotactic response of worms (Fig. 5). Additionally, (-)- gossypol did not alter the locomotory behavior of worms, which could influence their response towards the chemoattractant diacetyl. However, in mammals, high concentrations of free gossypol have been shown to cause respiratory distress, impaired body weight gain, weakness, apathy, impaired reproduction, compromised immune function and even death [30]. Thus, gossypol toxicity constitutes a strong limitation when it comes to using it as a pharmacological inhibitor for treating forgetting in humans. However, a recent study by Lan et al., 2018 revealed gossypolone (Gn), a (-)- gossypol metabolite, as a 20-fold more potent disruptor of MSI activity [39]. This could address the issue of toxicity discussed previously, since much lower concentrations would be required to achieve the same effect. Additionally, it should be pointed out, that the lack of toxicity in C. elegans does not preclude it in humans. Previous studies have shown how worm pharmacology can differ, where dopamine drugs had opposing effects on DA receptors in worms and mammals [40] and sodium azide is a permanent cytochrome c oxidase inhibitor in mammals whilst a reversible one in C. elegans. Thus, we acknowledge that caution must be paid for when using C. elegans as a model for drug screening.
Furthermore, (-)- gossypol has been shown not to be selective against MSI but can act on multiple pathways [41, 42]. However, we did not observe any behavioral changes in (-)- gossypol-treated msi-1(lf) worms. Moreover, (-)- gossypol treatment of worms expressing human MSI1 solely in the AVA interneuron inhibits the previously seen phenotypic rescue (Fig. 3), suggesting a tissue-specific mode of drug action rather than an off-target effect. Nevertheless, while the concentrations used in our treatment might not suffice to affect other pathways, we cannot exclude that (-)- gossypol could harbor off-target effects, as increased toxicity is observed with increasing concentrations of the drug. Future efforts should, therefore, focus on elucidating compounds that selectively target and inhibit MSI1/2.
Finally, physiological age-dependent cognitive decline normally observed in wild-type worms did not occur in 2-day-old msi-1(lf) worms or 2-day-old wild-type worms treated with (-)- gossypol. These results implicate Musashi in age-dependent memory decline and nominate Musashi-inhibiting compounds as memory modulators.
Taken together, our results argue in favor of an evolutionary conserved role for Musashi proteins in memory in species as diverse as nematodes and humans. Additionally, we show that C. elegans, similar to other model organisms [43], can be successfully used as a model for therapeutic intervention using pharmacological manipulations [44], which might prove useful in the treatment of memory-related disorders.
Data Availability
Not applicable.
References
Squire LR (1987) The organization and neural substrates of human memory. Int J Neurol 21–22:218–222
Wang S-H, Morris RGM (2010) Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61(1):49–79. https://doi.org/10.1146/annurev.psych.093008.100523
Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157(1):163–186. https://doi.org/10.1016/j.cell.2014.03.001
Kraemer PJ, Golding JM (1997) Adaptive forgetting in animals. Psychon Bull Rev 4(4):480–491. https://doi.org/10.3758/BF03214337
Davis RL, Zhong Y (2017) The biology of forgetting—a perspective. Neuron 95(3):490–503. https://doi.org/10.1016/j.neuron.2017.05.039
Ryan TJ, Frankland PW (2022) Forgetting as a form of adaptive engram cell plasticity. Nat Rev Neurosci 23(3):173–186. https://doi.org/10.1038/s41583-021-00548-3
Hadziselimovic N, Vukojevic V, Peter F, Milnik A, Fastenrath M, Fenyves BG, Hieber P, Demougin P et al (2014) Forgetting is regulated via musashi-mediated translational control of the Arp2/3 complex. Cell 156(6):1153–1166. https://doi.org/10.1016/j.cell.2014.01.054
Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T (2005) Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res 306(2):349–356. https://doi.org/10.1016/j.yexcr.2005.02.021
Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T et al (1996) Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol 176(2):230–242. https://doi.org/10.1006/dbio.1996.0130
Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H et al (2003) Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett 535(1–3):131–135. https://doi.org/10.1016/S0014-5793(02)03896-6
Colitti M, Farinacci M (2009) Expression of a putative stem cell marker, Musashi 1, in mammary glands of ewes. J Mol Histol 40(2):139–149. https://doi.org/10.1007/s10735-009-9224-3
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, Morgan K, Tam W et al (2010) Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med 16(8):903–908. https://doi.org/10.1038/nm.2187
Zearfoss NR, Deveau LM, Clingman CC, Schmidt E, Johnson ES, Massi F, Ryder SP (2014) A conserved three-nucleotide core motif defines Musashi RNA-binding specificity. J Biol Chem 289(51):35530–35541. https://doi.org/10.1074/jbc.M114.597112
Bennett CG, Riemondy K, Chapnick DA, Bunker E, Liu X, Kuersten S, Yi R (2016) Genome-wide analysis of Musashi-2 targets reveals novel functions in governing epithelial cell migration. Nucleic Acids Res 44(8):3788–3800. https://doi.org/10.1093/nar/gkw207
Karmakar S, Ramirez O, Paul KV, Gupta AK, Kumari V, Botti V, de los Mozos IR, Neuenkirchen N et al (2022) Integrative genome-wide analysis reveals EIF3A as a key downstream regulator of translational repressor protein Musashi 2 (MSI2). NAR Cancer 4(2):zcac015. https://doi.org/10.1093/narcan/zcac015
Lukong KE, Chang K, Khandjian EW, Richard S (2008) RNA-binding proteins in human genetic disease. Trends Genet 24(8):416–425. https://doi.org/10.1016/j.tig.2008.05.004
Darnell RB (2010) HITS-CLIP: Panoramic views of protein–RNA regulation in living cells. Wiley Interdisc Rev: RNA 1(2):266–286. https://doi.org/10.1002/wrna.31
Clingman CC, Deveau LM, Hay SA, Genga RM, Shandilya SM, Massi F, Ryder SP (2014) Allosteric inhibition of a stem cell RNA-binding protein by an intermediary metabolite. Elife 3:e02848. https://doi.org/10.7554/eLife.02848
Lan L, Appelman C, Smith AR, Yu J, Larsen S, Marquez RT, Liu H, Wu X et al (2015) Natural product (−)-gossypol inhibits colon cancer cell growth by targeting RNA-binding protein Musashi-1. Mol Oncol 9(7):1406–1420. https://doi.org/10.1016/j.molonc.2015.03.014
Colbert HA, Bargmann CI (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14(4):803–812. https://doi.org/10.1016/0896-6273(95)90224-4
Sengupta P, Chou JH, Bargmann CI (1996) Odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84(6):899–909. https://doi.org/10.1016/S0092-8674(00)81068-5
Yoda A, Sawa H, Okano H (2000) MSI-1, a neural RNA-binding protein, is involved in male mating behaviour in Caenorhabditis elegans. Genes Cells 5(11):885–895. https://doi.org/10.1046/j.1365-2443.2000.00378.x
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94. https://doi.org/10.1093/genetics/77.1.71
Bargmann CI, Hartwieg E, Horvitz HR (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74(3):515–527. https://doi.org/10.1016/0092-8674(93)80053-H
Nuttley WM, Atkinson-Leadbeater KP, van der Kooy D (2002) Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci 99(19):12449–12454. https://doi.org/10.1073/pnas.192101699
Vukojevic V, Gschwind L, Vogler C, Demougin P, de Quervain DJ-F, Papassotiropoulos A, Stetak A (2012) A role for α-adducin (ADD-1) in nematode and human memory: α-Adducin regulates synaptic plasticity. EMBO J 31(6):1453–1466. https://doi.org/10.1038/emboj.2012.14
Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B (2015) Streamlined genome engineering with a self-excising drug selection cassette. Genetics 200(4):1035–1049. https://doi.org/10.1534/genetics.115.178335
Dickinson DJ, Ward JD, Reiner DJ, Goldstein B (2013) Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods 10(10):1028–1034. https://doi.org/10.1038/nmeth.2641
Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10(12):3959–3970
Gadelha ICN, Fonseca NBS, Oloris SCS, Melo MM, Soto-Blanco B (2014) Gossypol toxicity from cottonseed products. Sci World J 2014:1–11. https://doi.org/10.1155/2014/231635
Oliver CL (2004) In vitro Effects of the BH3 Mimetic, (-)-Gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res 10(22):7757–7763. https://doi.org/10.1158/1078-0432.CCR-04-0551
Lian J, Ni Z, Dai X, Su C, Smith AR, Xu L, He F (2012) Sorafenib sensitizes (-)-gossypol-induced growth suppression in androgen-independent prostate cancer cells via Mcl-1 inhibition and Bak activation. Mol Cancer Ther 11(2):416–426. https://doi.org/10.1158/1535-7163.MCT-11-0559
Yang Z, Lan L, Wu X, Xu L, Buechner M (2019) RNA-binding proteins MSI-1 (Musashi) and EXC-7 (HuR) regulate serotonin-mediated behaviors in C. elegans. BioRxiv 748509. https://doi.org/10.1101/748509
Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT (2010) Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol 8(5):e1000372. https://doi.org/10.1371/journal.pbio.1000372
Ohyama T, Nagata T, Tsuda K, Kobayashi N, Imai T, Okano H, Yamazaki T, Katahira M (2012) Structure of Musashi1 in a complex with target RNA: The role of aromatic stacking interactions. Nucleic Acids Res 40(7):3218–3231. https://doi.org/10.1093/nar/gkr1139
Wu D (1989) An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs 38(3):333–341. https://doi.org/10.2165/00003495-198938030-00001
Yu-Wen Y (1987) Probing into the mechanism of action, metabolism and toxicity of gossypol by studying its (+)- and (−)-stereoisomers. J Ethnopharmacol 20(1):65–78. https://doi.org/10.1016/0378-8741(87)90120-6
Bailey CA, Stipanovic RD, Ziehr MS, Haq AU, Sattar M, Kubena LF, Kim HL, and de Vieira M R (2000) Cottonseed with a high (+)- to (−)-gossypol enantiomer ratio favorable to broiler production. J Agric Food Chem 48(11):5692–5695. https://doi.org/10.1021/jf000211n
Lan L, Liu H, Smith AR, Appelman C, Yu J, Larsen S, Marquez RT, Wu X et al (2018) Natural product derivative Gossypolone inhibits Musashi family of RNA-binding proteins. BMC Cancer 18(1):809. https://doi.org/10.1186/s12885-018-4704-z
Suo S, Ishiura S, Van Tol HHM (2004) Dopamine receptors in C. elegans. European J Pharmacol 500(1–3):159–166. https://doi.org/10.1016/j.ejphar.2004.07.021
Cheng J-S, Lo Y-K, Yeh J-H, Cheng H-H, Liu C-P, Chen W-C, Jan C-R (2003) Effect of gossypol on intracellular Ca2+ regulation in human hepatoma cells. Chin J Physiol 46(3):117–122
Wang J, Jin L, Li X, Deng H, Chen Y, Lian Q, Ge R, Deng H (2013) Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol BioSyst 9(6):1489. https://doi.org/10.1039/c3mb25461e
Papanikolopoulou K, Mudher A, Skoulakis E (2019) An assessment of the translational relevance of Drosophila in drug discovery. Expert Opin Drug Discov 14(3):303–313. https://doi.org/10.1080/17460441.2019.1569624
Giunti S, Andersen N, Rayes D, De Rosa MJ (2021) Drug discovery: insights from the invertebrate Caenorhabditis elegans. Pharmacol Res Perspect 9(2). https://doi.org/10.1002/prp2.721
Acknowledgements
We are grateful to Anne Spang for generously sharing reagents and instruments. We would also like to thank the Caenorhabditis Genetic Center (supported by NIH-NCRR) for providing nematode strains. We would finally like to thank the Imaging Core Facility of the Biozentrum (University of Basel) for microscopy support.
Funding
Open access funding provided by University of Basel. The work was supported by the Swiss National Science Foundation (SNSF) grant (31003A_156579).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by [Pavlina Mastrandreas], [Andreas Arnold] and [Attila Stetak]. The first draft of the manuscript was written by [Pavlina Mastrandreas] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Attila Stetak and Andreas Papassotiropoulos are co-senior authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mastrandreas, P., Arnold, A., Boglari, C. et al. (-)- Gossypol Inhibition of Musashi-Mediated Forgetting Improves Memory and Age-Dependent Memory Decline in Caenorhabditis elegans. Mol Neurobiol 60, 820–835 (2023). https://doi.org/10.1007/s12035-022-03116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03116-7