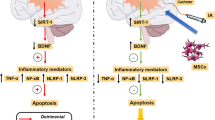
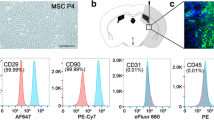
Abstract
Post-stroke edema and upregulation of aquaporin 4 (AQP4) water transport channels play a significant role in the progression of stroke pathology and deteriorating stroke outcomes. Prior studies from our lab have demonstrated the safety and efficacy of intra-arterial (IA) 1 × 105 mesenchymal stem cells (MSCs) administration post-stroke towards functional restoration and neuroprotection. Protein kinases have been reported to be involved in the signaling cascade of edema, with evidence supporting both its upregulation and downregulation at 24 h post-stroke. Among different protein kinase C (PKC) isoforms, the δ isoform is widely reported to play a pivotal role in the progression of ischemic reperfusion injury. Our present study aims to decipher the molecular mechanism of post-stroke IA MSCs mediated alleviation of perifocal vasogenic edema by PKCδ-mediated AQP4 regulation. Ovariectomized female SD rats were infused with 1 × 105 IA MSCs at 6 h post middle cerebral artery occlusion (MCAo). Animals were evaluated for behavioral and functional outcomes. Brains were harvested for evaluating infarct size and brain edema. Further, brain tissues were used for biochemical and molecular studies to decipher the possible molecular mechanism related to the regulation of PKCδ-mediated AQP4 expression. 1 × 105 IA MSCs at 6 h post-stroke confers neuroprotection as evident by the reduction in infarct size, edema, and improvement of functional outcome. An increase in GSH and catalase and a reduction in nitrite and MDA were observed along with a decrease in AQP4 and PKCδ expressions within the cortical brain regions of IA MSC–infused animals. The study gives preliminary evidence that IA MSCs administration post-stroke modulates PKCδ to regulate AQP4 expression which alleviates vasogenic edema towards neuroprotection. The study is novel and clinically relevant as no previous studies have looked into this aspect following IA delivery of stem cells in an animal model of ischemic stroke.

adapted from Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com/.). B Summary of the experimental design











Similar content being viewed by others
Data Availability
Available upon reasonable request from the corresponding author.
Code Availability
NA.
References
Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, Rangel EB, McNiece I, Rundek T, Sacco RL, Perez-Pinzon M, Hare JM (2014) Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 9(5):e93735. https://doi.org/10.1371/journal.pone.0093735
Chrostek MR, Fellows EG, Crane AT, Grande AW, Low WC (2019) Efficacy of stem cell-based therapies for stroke. Brain research 1722:146362
Dostovic Z, Dostovic E, Smajlovic D, Ibrahimagic OC, Avdic L (2016) Brain edema after ischaemic stroke. Med Arch 70(5):339–341. https://doi.org/10.5455/medarh.2016.70.339-341
Lindsay MP, Norrving B, Sacco RL, Brainin M, Hacke W, Martins S, Pandian J, Feigin V (2019) World Stroke Organization (WSO): Global Stroke Fact Sheet 2019. SAGE Publications Sage UK, London, England
Mamtilahun M, Tang G, Zhang Z, Wang Y, Tang Y, Yang GY (2019) Targeting water in the brain: role of aquaporin-4 in ischemic brain edema. Curr Drug Targets 20(7):748–755. https://doi.org/10.2174/1389450120666190214115309
Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci 9:108. https://doi.org/10.3389/fncel.2015.00108
Stokum JA, Mehta RI, Ivanova S, Yu E, Gerzanich V, Simard JM (2015) Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol Commun 3(1):61. https://doi.org/10.1186/s40478-015-0239-6
Hirt L, Fukuda AM, Ambadipudi K, Rashid F, Binder D, Verkman A, Ashwal S, Obenaus A, Badaut J (2017) Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab 37(1):277–290. https://doi.org/10.1177/0271678X15623290
Appunni S, Gupta D, Rubens M, Ramamoorthy V, Singh HN, Swarup V (2021) Deregulated protein kinases: friend and foe in ischemic stroke. Molecular Neurobiology:1–19
Bright R, Mochly-Rosen D (2005) The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 36(12):2781–2790. https://doi.org/10.1161/01.STR.0000189996.71237.f7
Zhao EY, Efendizade A, Cai L, Ding Y (2016) The role of Akt (protein kinase B) and protein kinase C in ischemia–reperfusion injury. J Neurol Res 38(4):301–308
Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D (2004) Protein kinase C δ mediates cerebral reperfusion injury in vivo. J Neurosci 24(31):6880–6888
Tang Y, Soroush F, Sun S, Liverani E, Langston JC, Yang Q, Kilpatrick LE, Kiani MF (2018) Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. J Neuroinflammation 15(1):309. https://doi.org/10.1186/s12974-018-1342-y
Tang X, Wang H, Chen H, Sun S, Chen H, Pan R (2021) Protective effects of Astragalus membranaceus and ligustrazine on rat brain microvascular endothelial cell injury after oxygen-glucose deprivation/reoxygenation by suppressing the PKCδ/MARCKS pathway. Comb Chem High Throughput Screen 24(7):947–956
Fukuda AM, Badaut J (2012) Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 9(1):279. https://doi.org/10.1186/1742-2094-9-279
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17(1):463–516
Turner RJ, Sharp FR (2016) Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 10:56. https://doi.org/10.3389/fncel.2016.00056
Li S, Li Y, Huang S, Fan B, Wei J, Ouyang L, Chen Z, Jiang B (2020) Silencing matrix metalloproteinase 9 exerts a protective effect on astrocytes after oxygen-glucose deprivation and is correlated with suppression of aquaporin-4. Neurosci Lett 731:135047
Alavian F, Hajizadeh S, Bigdeli MR, Javan M (2012) The role of protein kinase C in ischemic tolerance induced by hyperoxia in rats with stroke. EXCLI J 11:188–197
Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, Hu X, Feng W (2018) Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transplant 27(12):1723–1730. https://doi.org/10.1177/0963689718806846
Andrzejewska A, Lukomska B, Janowski M (2019) Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 37(7):855–864. https://doi.org/10.1002/stem.3016
Banerjee S, Williamson DA, Habib N, Chataway J (2012) The potential benefit of stem cell therapy after stroke: an update. Vasc Health Risk Manag 8:569–580. https://doi.org/10.2147/VHRM.S25745
Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, Geissler S, Reinke P (2019) Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med 25(2):149–163. https://doi.org/10.1016/j.molmed.2018.12.006
Sarmah D, Kaur H, Saraf J, Pravalika K, Goswami A, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P (2018) Getting closer to an effective intervention of ischemic stroke: the big promise of stem cell. Transl Stroke Res 9(4):356–374. https://doi.org/10.1007/s12975-017-0580-0
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23(9):1045–1059. https://doi.org/10.3727/096368913X667709
Kaur H, Sarmah D, Veeresh P, Datta A, Kalia K, Borah A, Yavagal DR, Bhattacharya P (2021) Endovascular stem cell therapy post stroke rescues neurons from endoplasmic reticulum stress-induced apoptosis by modulating brain-derived neurotrophic factor/tropomyosin receptor kinase B signaling. ACS Chem Neurosci 12(19):3745–3759. https://doi.org/10.1021/acschemneuro.1c00506
Saraf J, Sarmah D, Vats K, Kaur H, Pravalika K, Wanve M, Kalia K, Borah A, Dave KR, Yavagal DR, Bhattacharya P (2019) Intra-arterial stem cell therapy modulates neuronal calcineurin and confers neuroprotection after ischemic stroke. Int J Neurosci 129(10):1039–1044. https://doi.org/10.1080/00207454.2019.1633315
Vats K, Sarmah D, Datta A, Saraf J, Kaur H, Pravalika K, Wanve M, Kalia K, Borah A, Dave KR (2021) Intra-arterial stem cell therapy diminishes inflammasome activation after ischemic stroke: a possible role of acid sensing ion channel 1a. J Mol Neurosci 71(2):419–426
Sarmah D, Datta A, Kaur H, Kalia K, Borah A, Rodriguez AM, Yavagal DR, Bhattacharya P (2022) Sirtuin-1 - mediated NF-κB pathway modulation to mitigate inflammasome signaling and cellular apoptosis is one of the neuroprotective effects of intra-arterial mesenchymal stem cell therapy following ischemic stroke. Stem Cell Rev. https://doi.org/10.1007/s12015-021-10315-7
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Pravalika K, Sarmah D, Kaur H, Vats K, Saraf J, Wanve M, Kalia K, Borah A, Yavagal DR, Dave KR, Bhattacharya P (2019) Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci 216:49–58. https://doi.org/10.1016/j.lfs.2018.11.014
Wali B, Ishrat T, Atif F, Hua F, Stein DG, Sayeed I (2012) Glibenclamide administration attenuates infarct volume, hemispheric swelling, and functional impairments following permanent focal cerebral ischemia in rats. Stroke Res Treat 2012:460909. https://doi.org/10.1155/2012/460909
Kuts R, Frank D, Gruenbaum BF, Grinshpun J, Melamed I, Knyazer B, Tarabrin O, Zvenigorodsky V, Shelef I, Zlotnik A, Boyko M (2019) A novel method for assessing cerebral edema, infarcted zone and blood-brain barrier breakdown in a single post-stroke rodent brain. Front Neurosci 13:1105. https://doi.org/10.3389/fnins.2019.01105
Popp A, Jaenisch N, Witte OW, Frahm C (2009) Identification of ischemic regions in a rat model of stroke. PLoS ONE 4(3):e4764. https://doi.org/10.1371/journal.pone.0004764
Zhang J, Xiong H (2014) Brain tissue preparation, sectioning, and staining. In: Current laboratory methods in neuroscience research. Springer, pp 3–30
Granger DL, Taintor RR, Boockvar KS, Hibbs Jr JB (1996) Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. In: Methods in enzymology, vol 268. Elsevier, pp 142–151
HAS Syndromes drépanocytaires majeurs de l'enfant et de l'adolescent. Haute Autorité de Santé. http://www.has-sante.fr/portail/jcms/c_9o38888/ald-n-10-pnds-sur-syndromes-drepanocytaires-majeurs-de-l-enfant-et-de-l-adolescent
Todorović A, Pejić S, Stojiljković V, Gavrilović L, Popović N, Pavlović I, Saičić ZS, Pajović SB (2015) Antioxidative enzymes in irradiated rat brain—indicators of different regional radiosensitivity. Childs Nerv Syst 31(12):2249–2256
Li Y, Schellhorn HE (2007) Rapid kinetic microassay for catalase activity. Journal of biomolecular techniques: JBT 18(4):185
Aebi H (1984). Catalase in vitro. In: Methods in enzymology, vol 105. Elsevier, pp 121–126
Morimoto N, Oida Y, Shimazawa M, Miura M, Kudo T, Imaizumi K, Hara H (2007) Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience 147(4):957–967. https://doi.org/10.1016/j.neuroscience.2007.04.017
Yang B, Zador Z, Verkman AS (2008) Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem 283(22):15280–15286. https://doi.org/10.1074/jbc.M801425200
Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4(9):429–434. https://doi.org/10.4103/1947-2714.100998
Evilsizor MN, Ray-Jones HF, Lifshitz J, Ziebell J (2015) Primer for immunohistochemistry on cryosectioned rat brain tissue: example staining for microglia and neurons. J Vis Exp 99:e52293. https://doi.org/10.3791/52293
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B (2014) Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int 68:18–27. https://doi.org/10.1016/j.neuint.2014.02.001
Lenth RV (2006). Java applets for power and sample size. http://www.stat.uiowa.edu/~rlenth/Power
Yao Y, Zhang Y, Liao X, Yang R, Lei Y, Luo J (2020) Potential therapies for cerebral edema after ischemic stroke: a mini review. Front Aging Neurosci 12:618819. https://doi.org/10.3389/fnagi.2020.618819
IST-3 Collaborative Group (2012) The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 379(9834):2352–2363
Lin T-C, Lee J-D, Lin Y-H, Yuan R-Y, Weng H-H, Huang Y-C, Lee M, Wu C-Y, Hsu H-L, Hsu C-Y (2017) Timing of symptomatic infarct swelling following intravenous thrombolysis in acute middle cerebral artery infarction: a case–control study. Clin Appl Thromb Hemost 23(7):814–820
Li W, Shi L, Hu B, Hong Y, Zhang H, Li X, Zhang Y (2021) Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci 15:628940. https://doi.org/10.3389/fncel.2021.628940
Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, Moll G, Cox CS Jr (2019) Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol 10:1645. https://doi.org/10.3389/fimmu.2019.01645
Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW (2008) Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39(5):1569–1574. https://doi.org/10.1161/STROKEAHA.107.502047
Zhang HL, Xie XF, Xiong YQ, Liu SM, Hu GZ, Cao WF, Wu XM (2018) Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res 1680:143–154. https://doi.org/10.1016/j.brainres.2017.12.017
Park YJ, Borlongan CV (2021) Recent advances in cell therapy for stroke. J Cereb Blood Flow Metab:271678X211026507. https://doi.org/10.1177/0271678X211026507
Michinaga S, Koyama Y (2015) Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci 16(5):9949–9975
Zador Z, Stiver S, Wang V, Manley GT (2009) Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol 190:159–170. https://doi.org/10.1007/978-3-540-79885-9_7
Luo ZW, Ovcjak A, Wong R, Yang BX, Feng ZP, Sun HS (2020) Drug development in targeting ion channels for brain edema. Acta Pharmacol Sin 41(10):1272–1288. https://doi.org/10.1038/s41401-020-00503-5
Papadopoulos MC, Manley GT, Krishna S, Verkman AS (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18(11):1291–1293. https://doi.org/10.1096/fj.04-1723fje
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. https://doi.org/10.3389/fneur.2013.00032
Bernardo-Castro S, Sousa JA, Brás A, Cecília C, Rodrigues B, Almendra L, Machado C, Santo G, Silva F, Ferreira L (2020) Pathophysiology of blood–brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front neurol 11:1605
Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, Xu N, Stetler RA, Zhang F, Liu X (2016) Rapid endothelial cytoskeletal reorganization enables early blood–brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 7(1):1–18
Asahi M, Wang X, Mori T, Sumii T, Jung J-C, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci 21(19):7724–7732
Gidday JM, Gasche YG, Copin J-C, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ 289(2):H558–H568
Lucke-Wold BP, Turner RC, Logsdon AF, Simpkins JW, Alkon DL, Smith KE, Chen YW, Tan Z, Huber JD, Rosen CL (2015) Common mechanisms of Alzheimer’s disease and ischemic stroke: the role of protein kinase C in the progression of age-related neurodegeneration. J Alzheimers Dis 43(3):711–724. https://doi.org/10.3233/JAD-141422
Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29(5):1020–1030. https://doi.org/10.1161/01.str.29.5.1020
Dong X, Song YN, Liu WG, Guo XL (2009) Mmp-9, a potential target for cerebral ischemic treatment. Curr Neuropharmacol 7(4):269–275. https://doi.org/10.2174/157015909790031157
Wang Z, Xue Y, Jiao H, Liu Y, Wang P (2012) Doxycycline-mediated protective effect against focal cerebral ischemia–reperfusion injury through the modulation of tight junctions and PKCδ signaling in rats. J Mol Neurosci 47(1):89–100
Santa-Cecília FV, Socias B, Ouidja MO, Sepulveda-Diaz JE, Acuna L, Silva RL, Michel PP, Del-Bel E, Cunha TM, Raisman-Vozari RJNr (2016) Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox Res 29(4):447–459
Balducci C, Forloni G (2019) Doxycycline for Alzheimer’s disease: fighting β-amyloid oligomers and neuroinflammation. Front Pharmacol 10:738
Song H, Fares M, Maguire KR, Siden A, Potacova Z (2014) Cytotoxic effects of tetracycline analogues (doxycycline, minocycline and COL-3) in acute myeloid leukemia HL-60 cells. PLoS ONE 9(12):e114457. https://doi.org/10.1371/journal.pone.0114457
Wang G, Yuan Y, Zhang J, Gao L, Tan X, Yang G, Lv X, Jin Y (2014) Roles of aquaporins and matrix metalloproteinases in mouse brain edema formation induced by subacute exposure to 1,2-dichloroethane. Neurotoxicol Teratol 44:105–112. https://doi.org/10.1016/j.ntt.2014.06.005
Yang JL, Mukda S, Chen SD (2018) Diverse roles of mitochondria in ischemic stroke. Redox Biol 16:263–275. https://doi.org/10.1016/j.redox.2018.03.002
Yang C, Hawkins KE, Dore S, Candelario-Jalil E (2019) Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 316(2):C135–C153. https://doi.org/10.1152/ajpcell.00136.2018
Calió ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA (2014) Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med 70:141–154
Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chen YL, Chai HT, Lin KC, Liu CF, Chang HW, Lee MS, Yip HK (2016) Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7(46):74537–74556. https://doi.org/10.18632/oncotarget.12902
Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D (2004) Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci 24(31):6880–6888. https://doi.org/10.1523/JNEUROSCI.4474-03.2004
Inagaki K, Churchill E, Mochly-Rosen D (2006) Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res 70(2):222–230
Pan R, Tang X, Wang H, Huang Y, Huang K, Ling S, Zhou M, Cai J, Chen H, Huang Y (2020) The combination of Astragalus membranaceus and ligustrazine protects against thrombolysis-induced hemorrhagic transformation through PKCδ/Marcks pathway in cerebral ischemia rats. Cell Transplant 29:0963689720946020
Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A (2009) Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab 29(10):1683–1694. https://doi.org/10.1038/jcbfm.2009.87
Gołąb P, Boguszewska-Czubara A, Kiełbus M, Kurzepa J (2014) The rtPA increases MMP-9 activity in serum during ischaemic stroke. Neurol Neurochir Pol 48(5):309–314
Hsieh HL, Wu CY, Yang CM (2008) Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-δ-dependent ERK/Elk-1 pathway in astrocytes. Glia 56(6):619–632
Lee T-H, Chen J-L, Liu P-S, Tsai M-M, Wang S-J, Hsieh H-L (2020) Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. J Neuroinflammation 17:1–13
Weng Y-C, Wang G, Messing RO, Chou W-H (2015) Identification of lipocalin-2 as a PKCδ phosphorylation substrate in neutrophils. J Biomed Sci 22(1):1–10
Nakamura H, Sasaki Y, Sasaki M, Kataoka-Sasaki Y, Oka S, Nakazaki M, Namioka T, Namioka A, Onodera R, Suzuki J, Nagahama H, Mikami T, Wanibuchi M, Kocsis JD, Honmou O (2019) Elevated brain derived neurotrophic factor levels in plasma reflect in vivo functional viability of infused mesenchymal stem cells for stroke in rats. J Neurosurg Sci 63(1):42–49. https://doi.org/10.23736/S0390-5616.17.03989-3
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS (2014) Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int 2014:129145. https://doi.org/10.1155/2014/129145
Scheper V, Schwieger J, Hamm A, Lenarz T, Hoffmann A (2019) BDNF-overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro. J Neurosci Res 97(11):1414–1429. https://doi.org/10.1002/jnr.24488
Sladojevic N, Stamatovic SM, Johnson AM, Choi J, Hu A, Dithmer S, Blasig IE, Keep RF, Andjelkovic AV (2019) Claudin-1-dependent destabilization of the blood–brain barrier in chronic stroke. J Neurosci 39(4):743–757
Witkowski M, Landmesser U, Rauch U (2016) Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med 26(4):297–303
Ekdahl KN, Teramura Y, Asif S, Jonsson N, Magnusson PU, Nilsson B (2015) Thromboinflammation in therapeutic medicine. Adv Exp Med Biol 865:3–17. https://doi.org/10.1007/978-3-319-18603-0_1
Machado CdV, Telles PDdS, Nascimento ILO (2013) Immunological characteristics of mesenchymal stem cells. Rev Bras Hematol Hemoter 35:62–67
Acknowledgements
The authors acknowledge the National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad administration for providing the facility and support for conducting this study. The authors would also like to acknowledge Ms. Monika Seervi, Dr. Shirish Bhatiya, Mr. Pramod Kumar Suthar, and Mr. Vishal Gupta for their support in the study.
Funding
This study was funded by the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India and the Indian Council of Medical Research (ICMR), New Delhi, for the senior research fellowship grant of Ms. Aishika Datta (45/13/2020-PHA/BMS) and the NanoBio project grant to Dr. Pallab Bhattacharya (34/5/2019-TF/Nano/BMS).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: PB. Data acquisition: AD, DS, HK, AC, and KLM. Analysis and interpretation of data: AD, DS, HK, AC, KK, AB, DY, and PB. Drafting and revision of the manuscript: AD, DS, KK, AB, DY, and PB. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No clinical study was conducted.
Consent to Participate
NA
Consent for Publication
All authors have given their consent for the publication of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Datta, A., Sarmah, D., Kaur, H. et al. Post-stroke Impairment of the Blood–Brain Barrier and Perifocal Vasogenic Edema Is Alleviated by Endovascular Mesenchymal Stem Cell Administration: Modulation of the PKCδ/MMP9/AQP4-Mediated Pathway. Mol Neurobiol 59, 2758–2775 (2022). https://doi.org/10.1007/s12035-022-02761-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02761-2